Why Do Drinks Stay Hot in Winter and Cool in Summer?
According to the second law of thermodynamics, heat always flows from a warmer body to a colder one, leading to an increase in entropy. This process is irreversible and ultimately leads to the achievement of thermodynamic equilibrium. The thermos is designed to minimize this heat transfer and delay the balancing process, which helps to maintain the temperature of the liquid inside the thermos for extended periods [1,2].
Construction of A Thermos

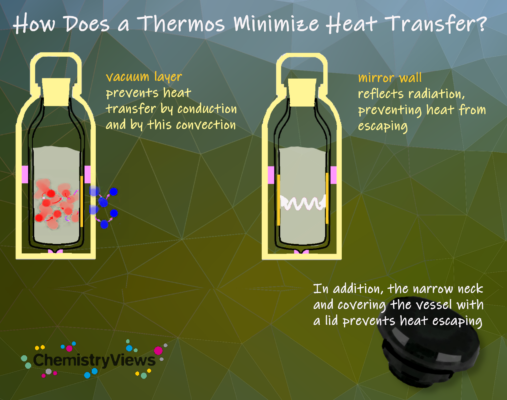
Today, a double-walled vessel made of stainless steel (high-grade steel) is often used in thermos flasks instead of the glass vessel. There is a vacuum between the inner and outer walls for insulation. This principle has somewhat poorer thermal insulation properties, but in everyday life, it is much less sensitive to shocks and sharp objects, for example when washing up.
The Three Possible Types of Heat Transfer
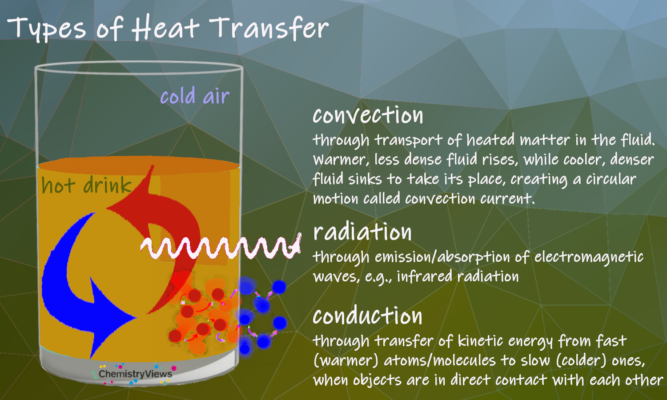
For the heat flow φ transferred by conduction, which occurs at a temperature gradient ΔT per unit of time between two (equally sized) surfaces A at a distance Δx, the following equation applies
φ = λ⋅A⋅ΔTΔx
the proportionality factor λ is called thermal conductivity or thermal conductivity coefficient.
The SI unit is W/(m K). The thermal conductivity of different materials varies widely. Here are the approximate thermal conductivity values for some materials:
- Glass: varies depending on the type of glass, but a typical value is around 1 W/(m K).
- Plastic: generally low, with values ranging from 0.1 to 0.5 W/(m K).
- Ceramic: varies depending on the type of ceramic, but values range from 1 to 5 W/(m K).
- Silver: is an excellent conductor of heat, with a thermal conductivity of approximately 430 W/(m K).
- Vacuum: has virtually no thermal conductivity since it is a space devoid of matter and therefore does not have any molecules that can transfer heat.
How Does This Work in the Thermos?
Is the Shape Important?

Brief Excursion into History
Still known as the Dewar flask among chemists, the vacuum flask is more widely known as the Thermos, named after the company that obtained the patent for the flask and to whom Dewar lost an ensuing court case [3,4].
 A Dewar vessel is a double-walled vessel made of glass or stainless steel that is evacuated and has a mirrored inner surface surface. It is used in thermos/insulated flasks and special laboratory containers. The vessel is named after Sir James Dewar (1842–1923; pictured on the right), a Scottish physicist and chemist who introduced vacuum vessels in 1872/73 and mirrored glass vessels as transport vessels for liquefied gases in 1893. It was expensive and time-consuming to liquefy gases; hence, Dewar designed a container where, once liquefied, gases could be kept for as long as possible. In 1881, Adolf Ferdinand Weinhold, a German physicist and chemist, also described a vacuum-jacketed bottle for laboratory purposes in his textbook “Physical Demonstrations.”
A Dewar vessel is a double-walled vessel made of glass or stainless steel that is evacuated and has a mirrored inner surface surface. It is used in thermos/insulated flasks and special laboratory containers. The vessel is named after Sir James Dewar (1842–1923; pictured on the right), a Scottish physicist and chemist who introduced vacuum vessels in 1872/73 and mirrored glass vessels as transport vessels for liquefied gases in 1893. It was expensive and time-consuming to liquefy gases; hence, Dewar designed a container where, once liquefied, gases could be kept for as long as possible. In 1881, Adolf Ferdinand Weinhold, a German physicist and chemist, also described a vacuum-jacketed bottle for laboratory purposes in his textbook “Physical Demonstrations.”
Reinhold Burger (1866–1954), a German glass technician who had worked for Dewar, developed double-walled vacuum glass vessels based on the Dewar vessel. He sent his vessels unsolicitedly to Carl von Linde (1842–1934), an ice machine manufacturer and later the founder of Linde plc. Carl von Linde commissioned Burger to supply him with insulating containers for transporting liquefied air at temperatures of –194.5 °C. Burger’s result was a double-walled, highly evacuated glass bulb, silver-plated on the inside, which was embedded in a lightweight metal wire container covered with felt on the inside for transport.
In 1903, Burger patented the thermos flask characterized by the mechanical stability of the inner vessel because of the support provided by inserts in the cavity and had the name “Thermos” protected as a trademark [9]. Together with his business partner Albert Aschenbrenner and the Viennese inventor and businessman Gustav Robert Paalen, Burger founded Thermos-Gesellschaft mbH in Berlin, Germany, in 1906, specifically for the production of thermos flasks. Thermos AG later became VEB Thermos. In 1906, Thermos GmbH transferred its foreign rights to the American Thermos Bottle Company in New York, USA, Thermos Limited, UK, and Thermos Bottle Co. Ltd. Canada.
Dewar lost a lawsuit against the thermos flask producer and Burger’s patent in the ensuing court battle. However, the name Dewar vessel remains till today.
Reference
[1] Katja Bammel, Thermoskanne – Vorsicht, heiß!, Spektrum der Wissenschaft Dezember 2009, 90–91.
[2] Dieter Meschede, Gerthsen Physik, 21. Aufl, Springer, Heildelberg, Germany, 2021.
[3] History and Heritage, Thermos website (accessed February 27, 2023)
[4] Robert J. Soulen, James Dewar, His Flask and Other Achievements, Physics Today 1996, 49(3), 32–37. https://doi.org/10.1063/1.881490
[5] Reinhold Burger, Gefaess mit doppelten, einen luftleeren Hohlraum einschliessenden Wandungen, Patent DE170057C 1909.
Also of Interest
Schlenk lines can be daunting for the first-time user. We looked at basic Schlenk line design and how to start and stop a Schlenk line

Exciting chemistry makes coffee so fascinating

The short video describes which processes take place in the body during alcohol breakdown and how long this takes




I have liked your creativity
Ok
I think it should have more talk about matreaals