The alcohol concentration in the breath is directly related to that in the blood. Due to its high water and fat solubility, ethanol can penetrate all cell membranes, and every organ in the body is flooded with dilute ethanol. As blood flows through the lungs, some of the alcohol passes through the membranes of the alveoli into the air, because alcohol is volatile and evaporates from a solution.
The concentration of alcohol in the alveolar air correlates with the blood alcohol concentration (the ratio of breath alcohol to blood alcohol is 1:2,100). This principle is used in breath alcohol testing devices. Instead of taking blood to test, e.g., a driver’s alcohol content, simply testing their breath is sufficient. If needed, a more accurate test can then be administered [1,2].
1 Different Forms of Breath Analyzers
Rolla Neil Harger (1890 – 1983), Indiana University School of Medicine, USA, is said to have developed the first method to measure alcohol content in the breath in 1931 and was awarded a patent in 1936, calling his device the drunkometer. It worked by simply relying on a color change, without any quantitative scale, due to a reaction between alcohol in the breath and acidified potassium permanganate [3].
1.1 Dichromate/Chromate Redox Reaction
In 1954, Robert Frank Borkenstein (1912 – 2002), a captain with the Indiana State Police and later a professor at Indiana University Bloomington, IN, USA, used chemical oxidation and photometry to determine the concentration of alcohol in breath. His trademarked breath analyzer was called Breathalizer and was based on the reaction between the alcohol in the breath of a test person and potassium dichromate [3].
In 1953, the German company Drüber developed an alcohol test that also relied on the redox reaction with dichromate. This method has been used for a very long time.
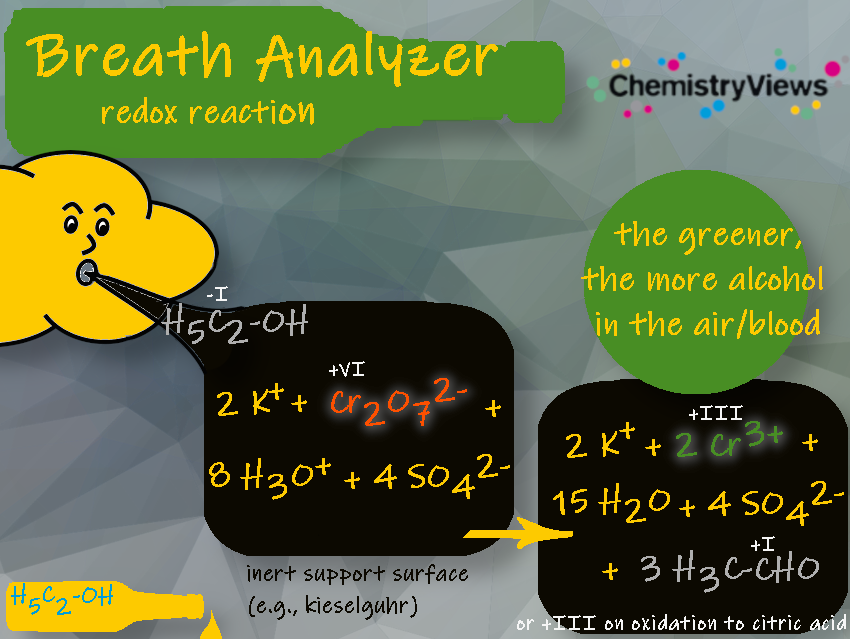
For detection, the system can be equipped with a photocell, a reference mixture, and a meter. The color difference generates an electric current in the photocells that moves a needle on the meter.
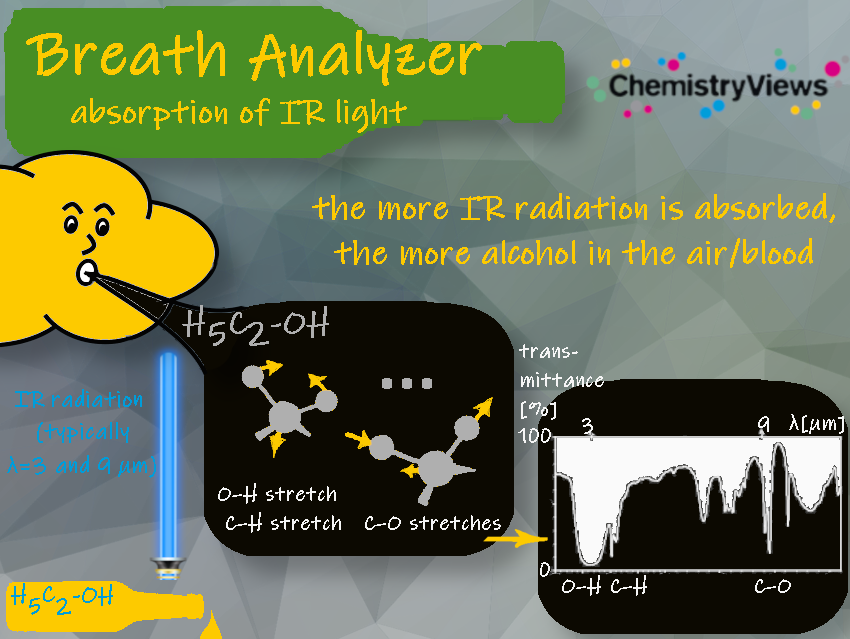
1.2 Absorption of IR Light
Each type of bond within a molecule leads to the absorption of IR light at different wavelengths. This leads to vibrational changes in the molecule. The wavelengths absorbed help identify the substance, and the intensity of IR absorption indicates the ethanol concentration.
1.3 Fuel-Cell Technology
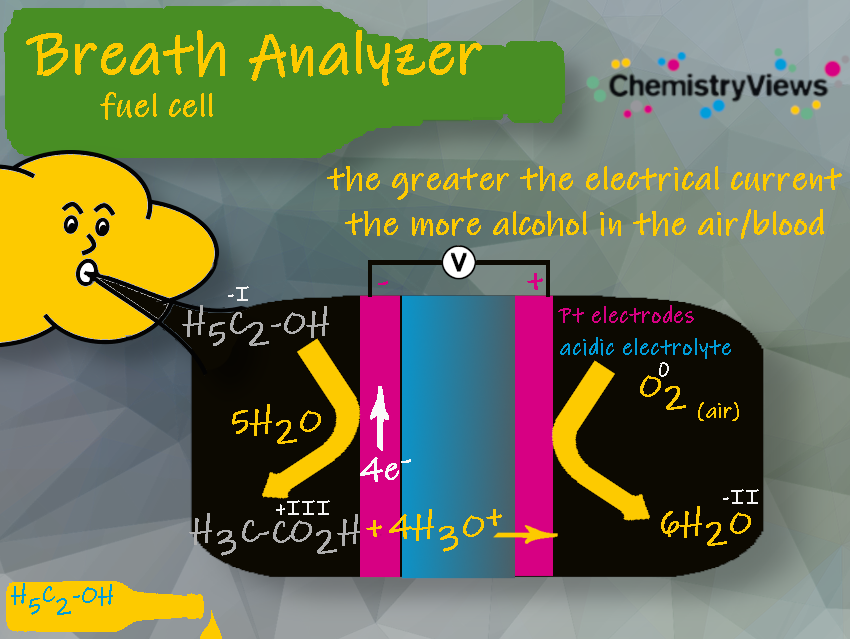
There are also other methods available. Breath analyzers may misinterpret elevated levels of acetone as ethanol. High acetone levels can occur in diabetics and those on fasting or ketogenic diets. To make the measurement highly specific to ethanol, the infrared and electrochemical measurements can be combined independently in one instrument.
2 Alcohol Degradation
The blood alcohol concentration is approximated by the Widmark formula [2,4]:
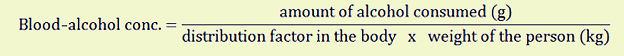
Erik Matteo Prochet Widmark (1889 – 1945) was a Swedish chemist and professor at the Institute of Medical Chemistry at the University of Lund, Sweden. In 1922, he developed the method for determining blood alcohol concentration. The distribution factor is different for men and women and depends on their respective body water content.
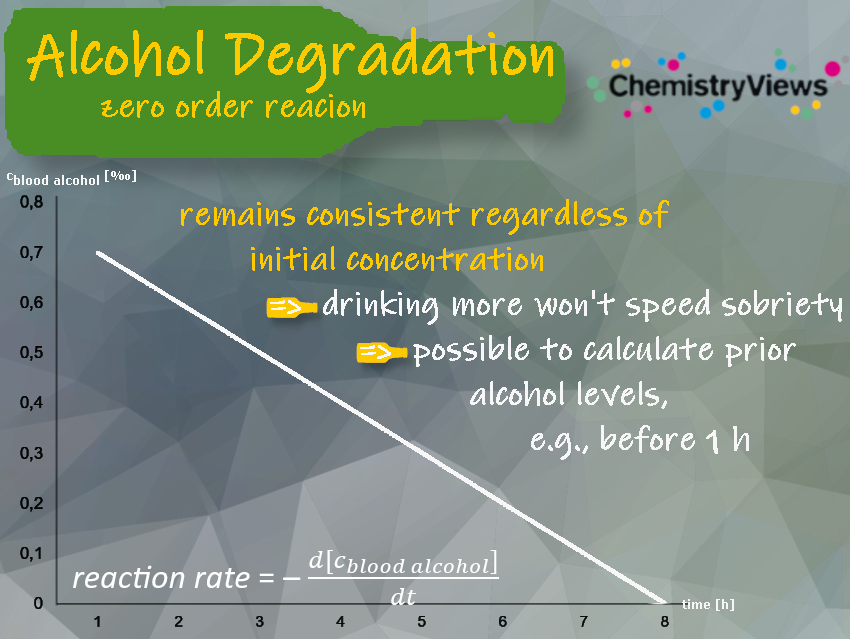
The reduction of the blood alcohol concentration or blood alcohol level follows a zero-order reaction with a constant rate of about 0.1 ‰ per hour as shown in the picture below [2,3]. You can learn more about the breakdown of alcohol in the body in the Clever Picture “From Sip to Slip: How Our Bodies Process Alcohol” [4].
References
[1] Erste Hilfe – Chemie und Physik für Mediziner (Eds: Jürgen Schatz, Robert Tammer), 2. Aufl. Springer, Heidelberg, Germany, 2021, pages 365, 379.
[2] Klaus Roth, Chemistry of a Hangover — Alcohol and its Consequences, ChemistryViews 2011. https://doi.org/10.1002/chemv.201000074
[3] Ada McVean, Before the Breathalyzer There Was the Drunkometer, McGill University, Montreal, Quebec, Canada, Jul 4. 2018. (accessed March 4, 2024)
[4] From Sip to Slip: How Our Bodies Process Alcohol, ChemistryViews 2013. https://doi.org/10.1002/chemv.201300091
Also of Interest

What happens to alcohol after it has been consumed?

How can a tiny molecule like ethanol be at the root of so much human misery?

A closer look at the chemical role hops play in the beer-brewing process shows how beer owes its marvelous flavor to a great deal of chemistry