Nanographenes with “defects” that form large macrocycles, i.e., porous nanographenes can have useful optical and electronic properties and can be useful, e.g., in host-guest chemistry. Porous nanographenes with complex structures can be difficult to synthesize in a controlled manner.
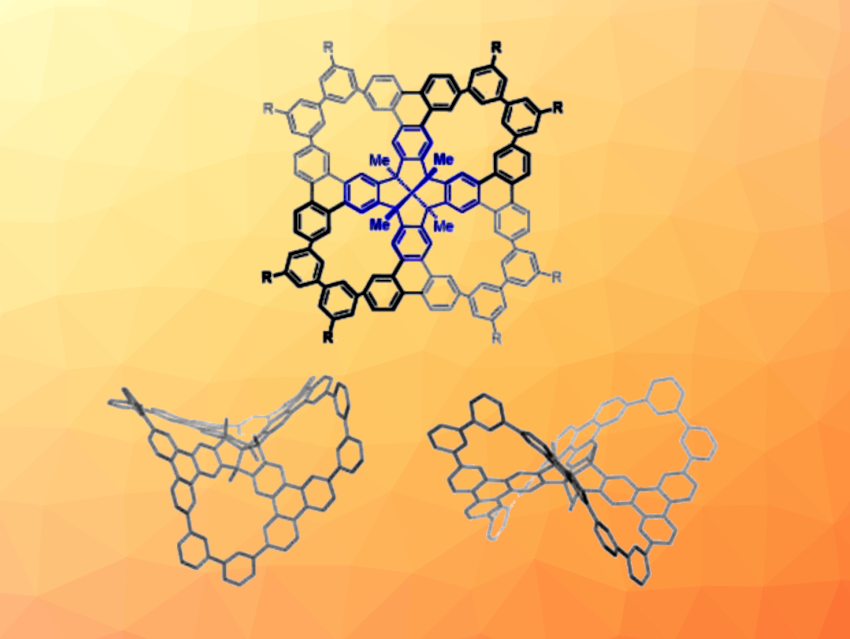
Dietmar Kuck, Bielefeld University, Germany, Hak-Fun Chow, The Chinese University of Hong Kong, Shatin, and colleagues have synthesized highly twisted porous nanographenes with a fenestrindane core (general structure pictured, fenestrindane core in blue). The team started from a fenestrindane derivative that is brominated in eight positions, which was subjected to an eightfold Suzuki-Miyaura coupling with 3-methoxyphenylboronic acid. This was followed by a fourfold Scholl cyclodehydrogenation and another eightfold Suzuki-Miyaura reaction to give precursors for the desired nanographenes. Finally, a fourfold intramolecular Yamamoto coupling gave the porous nanographenes in yields of 17–18 %.
The products (R = OnC6H13 or tBu) contain four macrocycles and have saddle-shaped structures with almost S4-symmetric conformations. According to the researchers, they represent the first porous nanographenes based on a [5.5.5.5]fenestrane motif. The substituents R in the periphery of the molecules improve their solubility in organic solvents. The absorption spectra of both products show only a small red shift compared with their non-cyclized precursors, which indicates that the cyclization process does not lead to significantly enhanced π-conjugation—a possible consequence of the highly twisted structure.
- Highly Twisted Fenestrindane‐Based Porous Nanographenes,
Xiao-Qing Sun, Yuke Li, Dietmar Kuck, Hak-Fun Chow,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202402931