The hydrosilylation of alkenes and alkynes is an important process in organosilicon chemistry, mostly catalyzed by platinum-based complexes. Replacing platinum-based catalysts with lower-cost, Earth-abundant elements in this type of transformation would be useful.
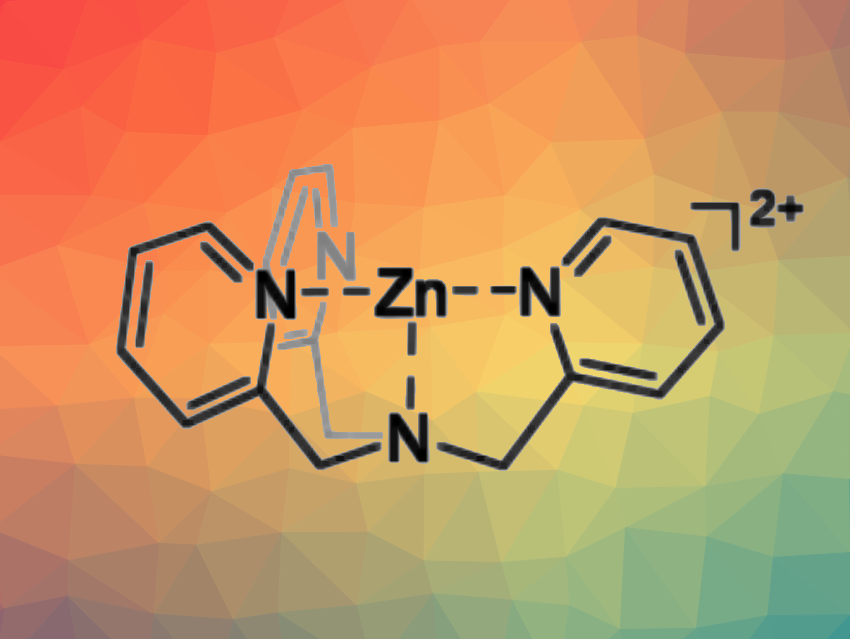
Roman Dobrovetsky, Tel Aviv University, Israel, and colleagues have synthesized a dicationic zinc complex with a tripodal tris(2-pyridylmethyl)amine (TPA) ligand by reacting TPA with Zn(OTf)2, followed by the addition of 2 equivalents of KB(C6F5)4. The resulting Zn-based complex (pictured above) was found to be an active hydrosilylation catalyst with a very high selectivity towards the hydrosilylation of alkynes.
Remarkably, when the team performed a reaction of Et3SiH with a 1:1 mixture of 1-hexene and phenylacetylene in the presence of 5 mol% of the catalyst, only the C≡C triple bond underwent hydrosilylation, while 1-hexene remained intact (simplified reaction pictured below). Mechanistic studies of this reaction revealed that unlike typical Zn-catalyzed hydrosilylations, which usually involve the activation of an Si–H bond, the key step in this case is the activation of the C≡C triple bond, which presumably is the reason for the observed selectivity.
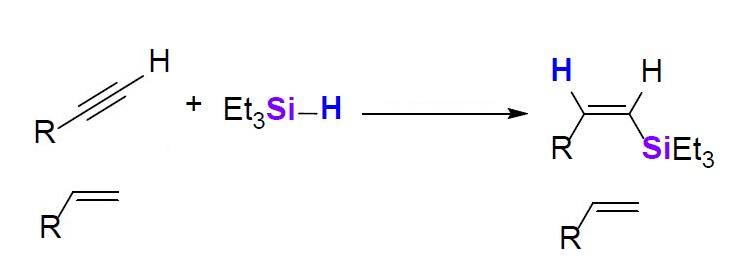
- Highly Chemoselective Zn2+‐Catalyzed Hydrosilylation of Alkynes,
Itay Raz, Roman Dobrovetsky,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202300798