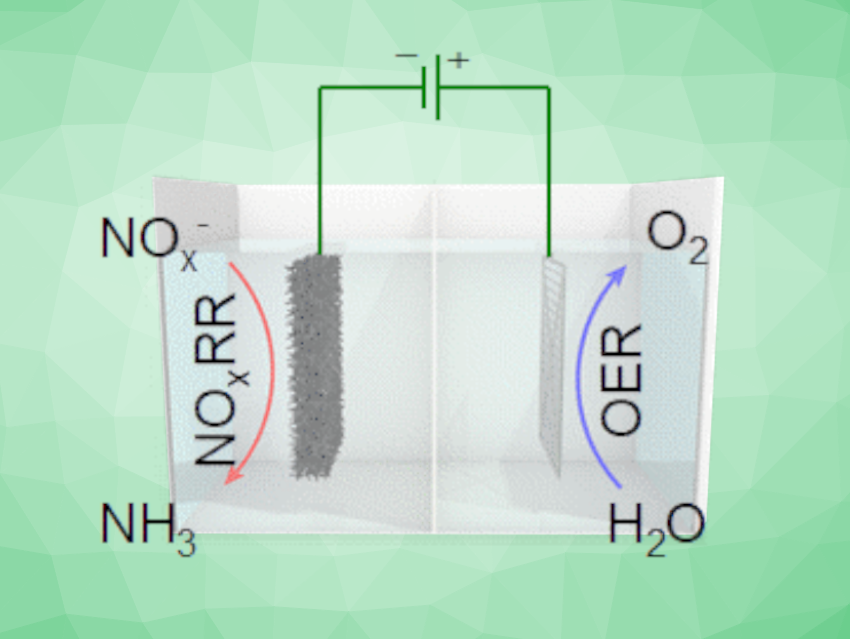
Ammonia is essential for the production of fertilizer, and thus, food. Its industrial-scale synthesis relies on the energy-intensive Haber–Bosch process, which contributes significantly to global carbon emissions. Alternative processes based on renewable electricity would, thus, be useful, but require suitable electrocatalysts. Using N2 as the nitrogen source in this approach can be difficult due to its low solubility and high bond strength. The electrochemical synthesis of NH3 via an NOx reduction reaction (NOxRR) is a promising alternative.
Hai-Xia Zhong, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Jun-Min Yan, Miao-Miao Shi, Jilin University, Changchun, China, and colleagues have developed an approach for the efficient electrochemical synthesis of ammonia from NOx on a copper-activated cobalt foam electrode. The team prepared the electrode material from a cobalt foam using a solution of copper nitrate and 2-methylimidazole (DMZ) to form a precatalyst, which was then activated by electrolysis in an alkaline electrolyte. This activation optimized the morphology of the electrode material and increased the active surface area.
The researchers used the developed electrode material in a bipolar membrane (BPM)-based electrolyzer for the reduction of nitrite. They observed a high NH3 selectivity with a Faradaic efficiency of up to ca. 96 % and high ammonia yield rates of ca. 279 mg h–1 cm–2. The electrode material showed good recyclability with no obvious degradation over 30 cycles. The team achieved NH3 production up to 4.11 g h–1 in a homemade reactor, which demonstrates the practical feasibility of the approach.
- Gram‐level NH3 Electrosynthesis via NOx reduction on Cu Activated Co Electrode,
Dong-Xue Liu, Zhe Meng, Yong-Fu Zhu, Xue-Feng Sun, Xin Deng, Miao-Miao Shi, Qi Hao, Xia Kang, Tian-Yi Dai, Hai-Xia Zhong, Junmin Yan, Qing Jiang,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202315238