Most solids expand as temperatures increase and shrink as they cool. Some materials do the opposite, expanding in the cold. Lithium titanium phosphate is one such substance. It could provide a solution to the problem of steeply declining performance of lithium-ion batteries in cold environments. Liming Wu, Inner Mongolia University, Hohhot, China, Chunfu Lin, Donghua University, Shanghai, China, Qingdao University, China, and Fudan University, Shanghai, China, Renchao Che, Donghua University and Fudan University, and colleagues have demonstrated its suitability for use in electrodes for rechargeable batteries.
Battery Performance at Low Temperatures
Lithium-ion batteries and other rechargeable batteries based on metal ions provide our portable devices with electricity, power vehicles, and store solar and wind energy. They work well—as long as it is warm. As temperatures drop, the performance of these batteries can decrease sharply—a problem for electric cars, aerospace, and military applications.
Countermeasures such as integrated heaters, improved electrolytes, or electrode coatings increase the cost and complexity of battery production or reduce performance. Therefore, new strategies capable of effectively and easily tackling the low-temperature issue of metal-ion batteries are desirable.
Negative Thermal Expansion
One of the causes of the cold problem is the slowed diffusion of lithium ions within the electrode material. The researchers proposed a new approach to tackling this issue: electrodes made of electrochemical energy-storage materials with negative thermal expansion (NTE), such as lithium titanium phosphate (LiTi2(PO4)3, LTP). The team used LTP as a model substance to demonstrate that electrode materials with NTE properties can provide good performance at low temperatures.
Analysis of the crystal structure revealed a three-dimensional lattice of TiO6 octahedra and PO4 tetrahedra with an open, flexible structure. The compound contains both “cavities” and “channels” where lithium ions can lodge.
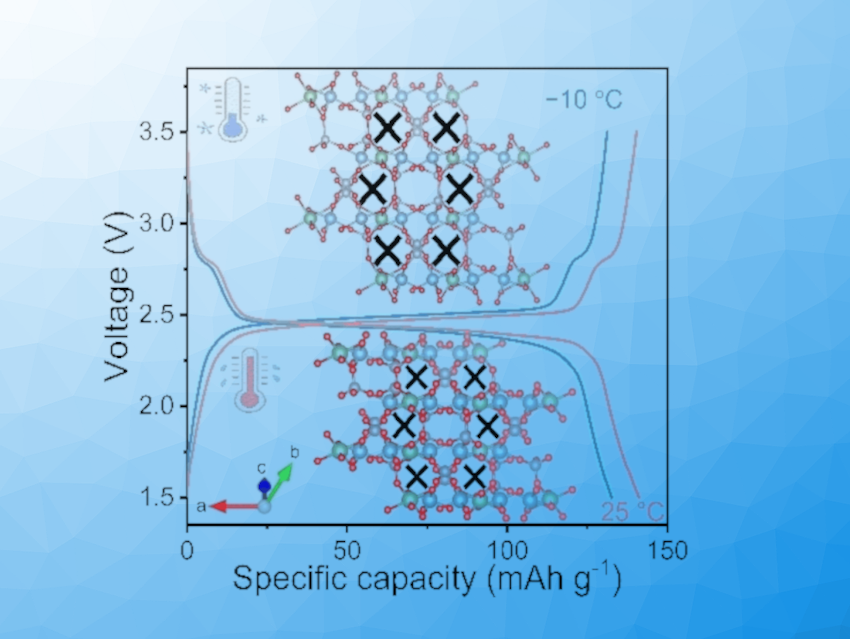
Better Diffusion, Improved Performance
When cooled, the structure stretches along one of its crystal axes. By using spectrometric and electron microscopic analyses in conjunction with computer modeling, the team determined that the vibrational modes of the atoms change at low temperature. This increases the occurrence of special transverse vibrations of certain oxygen atoms, increasing their distances from each other and widening the cavities in the lattice.
This facilitates the storage and transport of lithium ions. At −10 °C, their diffusion rate is still at 84 % of the value observed at 25 °C. Electrochemical tests on carbon-coated LTP at −10 °C also showed good electrochemical performance with high capacity and a high rate capability, as well as a high retention of capacity over 1000 charge/discharge cycles. Materials with negative thermal expansion are, thus, highly promising for use as an electrode material in lithium-ion batteries in cold environments.
- Negative Thermal Expansion Behavior Enabling Good Electrochemical‐Energy‐Storage Performance at Low Temperatures,
Qiao Li, Liting Yang, Guisheng Liang, Jiahui Yu, Shaowu Huang, Liming Wu, Chunfu Lin, Renchao Che,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202419300