The chlorination of small molecules can be interesting both for industrial applications and research. Most sulfur chlorides, for example, are relatively unstable and thus, can be challenging to synthesize. While both [SeCl6]2– and [TeCl6]2– have been synthesized, their lighter analogue [SCl6]2– had not been prepared so far.
Sebastian Hasenstab-Riedel and colleagues, Free University Berlin, Germany, have performed the first synthesis of this hexachloro sulfate(IV) dianion in the salt [NEt3Me]2[SCl6]. This compound was directly prepared in one step starting from elemental sulfur using the trichloride ionic liquid [NEt3Me][Cl3] (see below). This ionic liquid chlorinates and oxidizes the elemental sulfur, and the [NEt3Me]+ cations stabilize the [SCl6]2– anion.
![]()
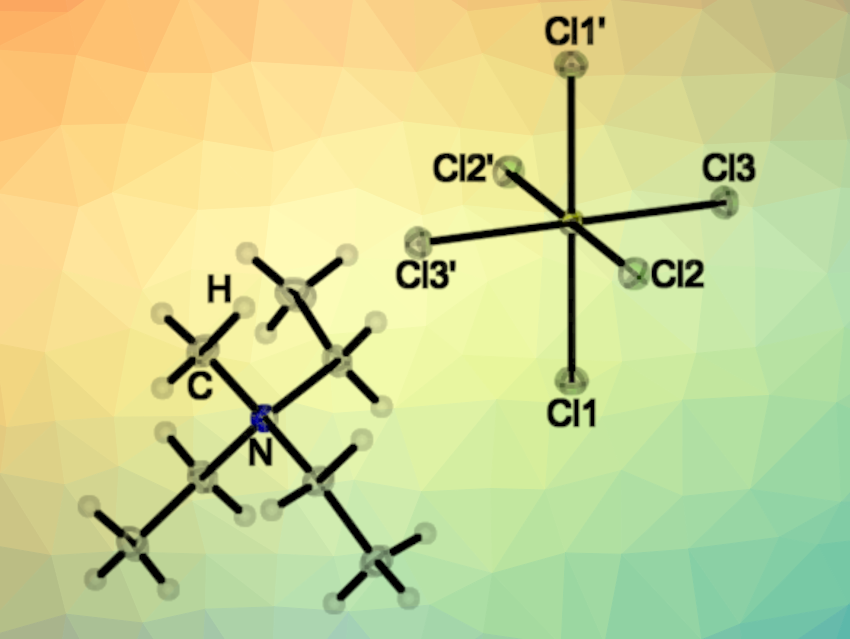
[SCl6]2– belongs to the AB6E systems, i.e., it has a central atom (A) with six “ligands” (B) and one lone pair (E). These systems should have a stereochemically active lone pair and adopt distorted octahedral structures, according to the valence shell electron pair repulsion (VSEPR) model. However, the VSEPR model is not always correct: For the anions [SeCl6]2– and [TeCl6]2–, both regular and distorted octahedral structures were found, depending on the cation. The team characterized [NEt3Me]2[SCl6] using X-ray crystallography (structure pictured at the top). They found that the [SCl6]2– dianion has an octahedral structure with a stereochemically inactive electron pair.
This work shows the ability of ionic liquids based on polychlorides to act as both an oxidizing and a stabilizing reagent and enable the synthesis of unprecedented molecules.
- Synthesis of a Hexachloro Sulfate(IV) Dianion Enabled by Polychloride Chemistry,
Patrick Voßnacker, Alisa Wüst, Carsten Müller, Merlin Kleoff, Sebastian Hasenstab-Riedel,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202209684