Magnesium-based Grignard reagents are widely used organometallic reagents. The underlying Schlenk equilibria (2 RMgX ⇌ MgX2 + MgR2), which determine their speciation in solution, have been well-studied. While related compounds have been investigated for heavier homologs of magnesium (Ca, Sr, and Ba), the lightest group 2 element, beryllium, has been neglected in comparison.
Magnus R. Buchner, University of Marburg, Germany, and colleagues have investigated the behavior of mixtures of beryllium halides and diphenyl beryllium in the N-and O-donor solvents NEt3 and tetrahydrofuen (THF). The mixtures were also studied in the non-coordinating solvents benzene and dichloromethane in the presence of donor ligands such as N-heterocyclic carbenes. The team performed NMR-spectroscopic analysis in combination with single-crystal X-ray diffraction and quantum-chemical calculations.
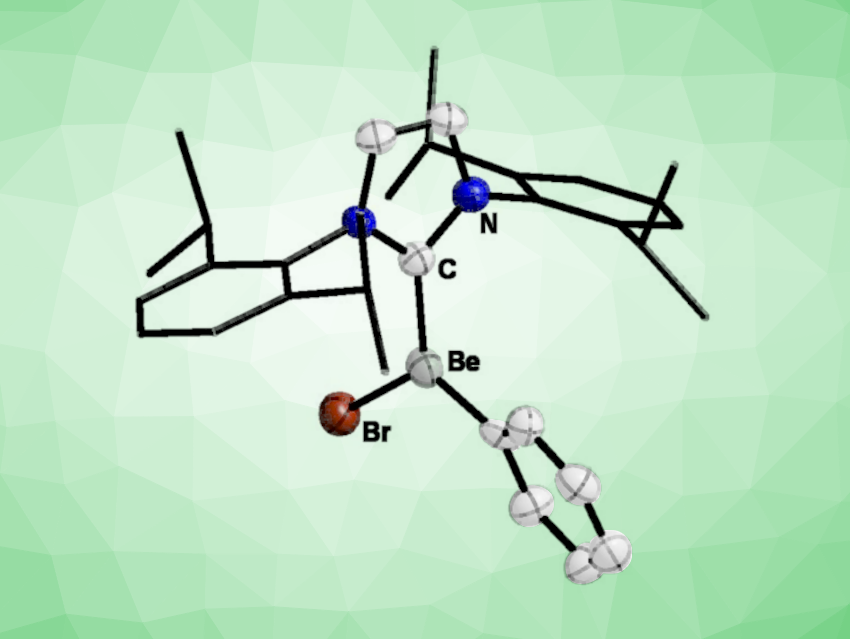
The researchers found that in all cases heteroleptic beryllium Grignard compounds of the general formula [(L)1-2BePhX]1-2 (X = Cl, Br, I; L = C-, N-, O-donor ligand) were formed selectively (example pictured). These beryllium Grignard compounds are closely related to the classic magnesium systems. However the beryllium complexes are more defined, very stable, and highly soluble in aromatic solvents and dichloromethane.
- A preference for heterolepticity – Schlenk type equilibria in organometallic beryllium systems,
Magnus Richard Buchner, Lewis R. Thomas-Hargreaves, Chantsalmaa Berthold, Deniz F. Bekiş, Sergei I. Ivlev,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302495