Dioxiranes, i.e., compounds with three-membered rings containing two oxygen atoms and one carbon atom, can be useful intermediates in organic synthesis. Trioxetanes, analogous four-membered rings with three oxygen atoms and a single carbon atom, have remained theoretical constructs so far. Some heavier chalcogen analogs of dioxiranes are known, but diseleniranes and ditelluriranes, as well as heavier chalcogen analogs of trioxetanes, have remained elusive. Formally replacing the carbon atom, too, to generate new compound classes is also possible, but examples of the resulting main group dichalcogeniranes or trichalcogenetanes are rare.
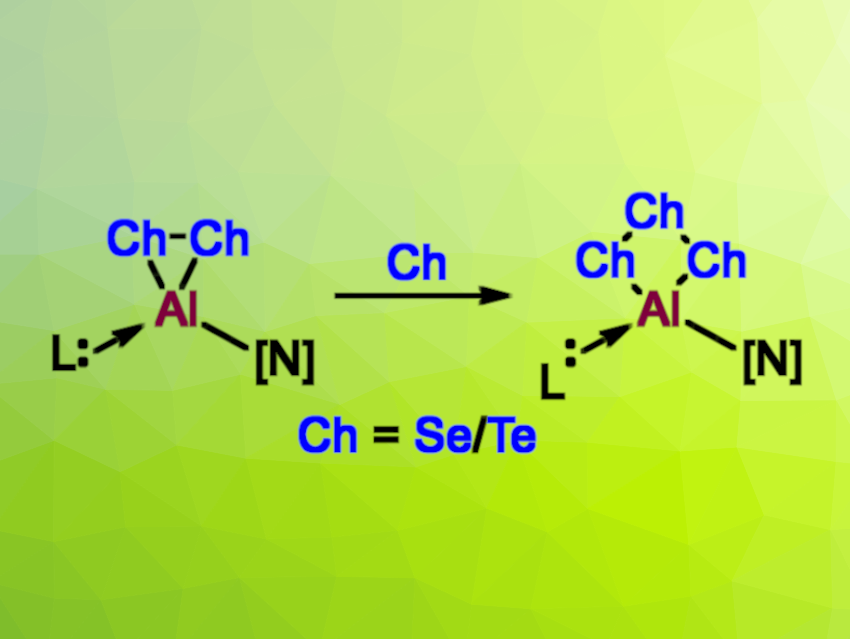
Lingbing Kong, Shandong University, Jinan, China, Liu Leo Liu, Southern University of Science and Technology, Shenzhen, China, and colleagues have synthesized heavier analogues of dioxiranes and trioxetanes incorporating aluminium and the chalcogens selenium or tellurium, i.e., aluminadichalcogeniranes and aluminatrichalcogenetanes (simplified structures pictured). Specifically, the team prepared an aluminadiselenirane (with an AlSe2 ring), an aluminaditellurirane (with an AlTe2 ring), an aluminatriselenetane (with an AlSe3 ring), an aluminatritelluretane (with an AlTe3 ring), and a mixed Se/Te analogue of an aluminatrichalcogenetane (with an AlSe2Te ring).
The researchers started from a base-stabilized aluminylene of the type [N]−Al(iPr2-bimy) with [N] = 1,8-bis(3,5-di-tert-butylphenyl)-3,6-di-tert-butylcarbazolyl and iPr2-bimy = 1,3-diisopropylbenzimidazole-2-ylidene, which was reacted with two equivalents of Se in toluene to give the aluminadiselenirane. A reaction of the same precursor with 2.1 equivalents of (nBu)3P=Te gave the aluminaditellurirane. From the aluminadiselenirane, the larger aluminatriselenetane could be synthesized via a reaction with another 1.2 equivalents of Se. Likewise, the aluminatritelluretane was obtained from the aluminaditellurirane by reacting it with 1.2 equivalents of Te. The mixed AlSe2Te-type product was obtained when the aluminadiselenirane was reacted with 1.2 equivalents of Te.
According to the researchers, the products feature high ring strains and significant polarization between the aluminum and chalcogen components, which leads to interesting reactivities and allowed the synthesis of several unprecedented main group heterocycles.
- Aluminadichalcogeniranes and Aluminatrichalcogenetanes: Heavier Analogs of Dioxiranes and Trioxetanes,
Xin Zhang, Lingbing Kong, Liu Leo Liu,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202422335