Poly(tetrafluoro)ethylene (PTFE) is widely used, e.g., in non-stick coatings or water-resistant materials. Perfluoroalkyl substances (PFAS) such as PTFE can remain in the environment for long periods of time and pose health hazards. PTFE is very stable, and there are limited options available to recycle or destroy it. The depolymerization of PTFE requires high temperatures and, thus, is energy-intensive. Defluorination could be a useful alternative approach, especially if the removed fluorine atoms can be used in further chemical syntheses.
Mark R. Crimmin, Imperial College London, UK, and colleagues have developed a method for the controlled defluorination of PTFE powder with a molecular magnesium-based reducing agent (pictured below on the left) at room temperature. The magnesium reactant was mixed with PTFE powder and 4-(dimethylamino)pyridine (DMAP) in benzene under an argon atmosphere.
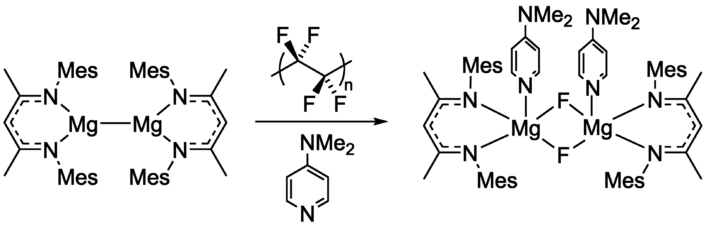
The team isolated a fluoride-bridged product (pictured above on the right) in a yield of 85 %. During the reaction, PTFE was converted from a colorless to a gray powder, and the researchers found evidence for the formation of C=C bonds. The fluoride-bridged, magnesium-containing product can be used as a fluorinating agent, thus allowing the reuse of the recovered F atoms.
- Room Temperature Defluorination of Poly(tetrafluoroethylene) by a Magnesium Reagent,
Daniel J. Sheldon, Joseph M. Parr, Mark R. Crimmin,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c02526
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

