Cyanosilylethers are useful building blocks for organic synthesis. There are various catalysts for the synthesis of these compounds, but many of them suffer from drawbacks such as a need for harsh reaction conditions, stability issues, or a limited substrate scope. Ruthenium-based complexes can catalyze a variety of organic reactions in a highly efficient and selective manner. They are also promising candidates for cyanosilylations.
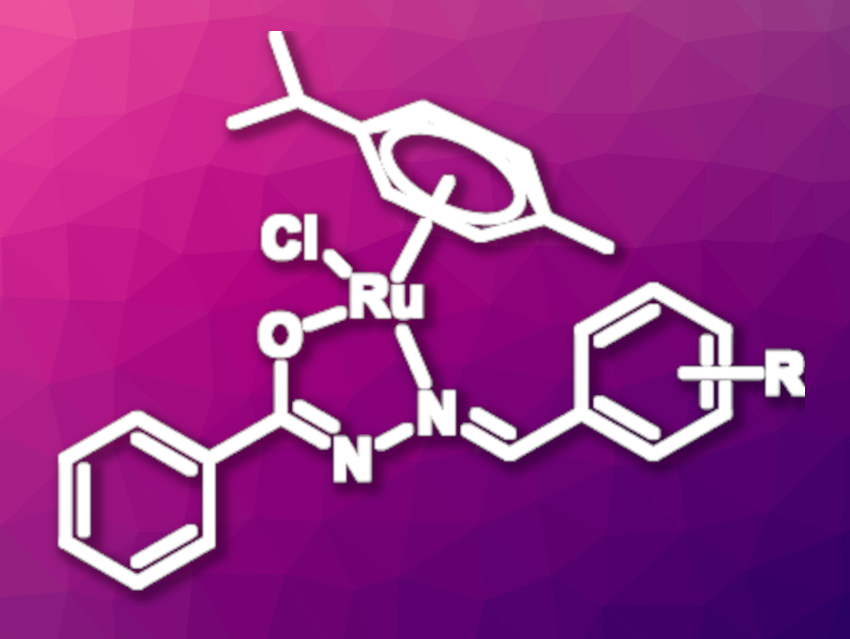
Zhen-Jiang Liu, Shanghai Institute of Technology, China, Zi-Jian Yao, Shanghai Institute of Technology and Anhui Normal University, Wuhu, China, and colleagues have developed a series of ruthenium half-sandwich complexes with a hydrazone ligand and an η6-bound p-cymene ligand (example pictured). The complexes were prepared from [(p-cymene)RuCl2]2 and the respective hydrazone ligand in methanol. The synthesis was carried out under inert conditions, but the isolated complexes are air- and moisture-stable.
The team chose benzaldehyde as a test substrate for cyanosilylation. It was reacted with trimethylsilyl cyanide at room temperature in dichloromethane in the presence of 0.1 mol% of the ruthenium catalyst. The target product was obtained in 94–95 % yield for all tested ruthenium catalysts after 1 h. Similarly high yields were also obtained for various aromatic aldehydes with electron-donating and electron-withdrawing groups, as well as heteroaryl aldehydes. The yields with aliphatic aldehydes as substrates were slightly lower, but still above 80 %. The approach was also used for the cyanosilylation of ketones, with yields of 85–93 %.
Overall, the developed ruthenium catalysts are stable, active at very loading under mild conditions, and useful for the conversion of a wide range of substrates. Therefore, they could be a useful addition to the toolbox of cyanosilylation catalysts.
- Half-Sandwich Ruthenium Complexes with Hydrazone Ligands: Preparation, Structure, and Catalytic Activity in Cyanosilylether Synthesis under an Air Atmosphere,
Ke Wang, Heng Li, Lin Yang, Zhen-Jiang Liu, Zi-Jian Yao,
Inorg. Chem. 2023.
https://doi.org/10.1021/acs.inorgchem.3c00819