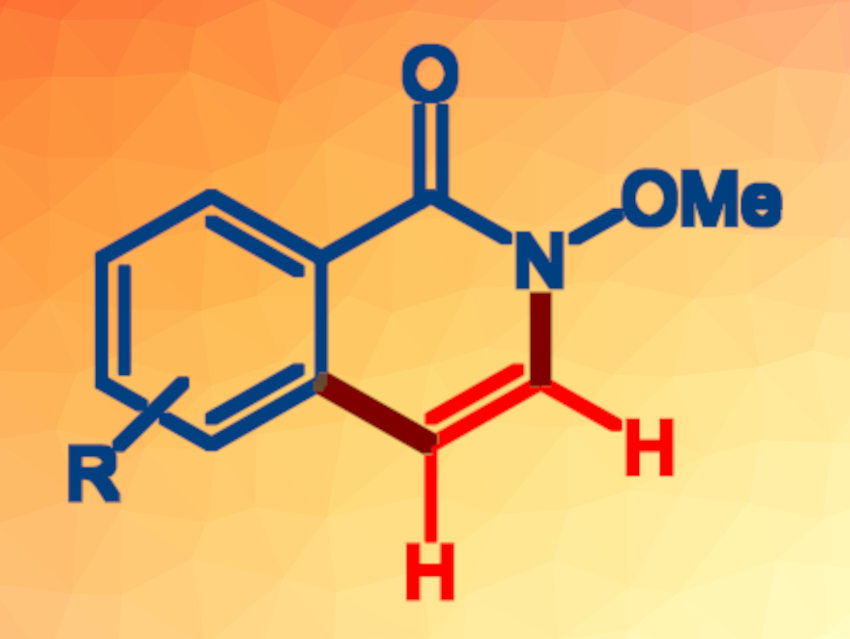
Isoquinolin-1(2H)-one is an important structural unit in natural products and bioactive compounds. Developing efficient methods for accessing isoquinolinones is, thus, an interesting research target. A transition-metal-catalyzed ortho-C‒H bond activation and [4+2] annulation using benzamides and internal alkynes is a promising route in this context, but this type of reaction can be challenging for 3,4-unsubstituted isoquinolones.
Parthasarathy Gandeepan, Indian Institute of Technology Tirupati, India, and colleagues have developed a mild and convenient synthesis of 3,4-unsubstituted isoquinolones from N-methoxybenzamides and vinylene carbonate, which acts as an acetylene surrogate (pictured below). The team used the commercially available rhodium(III) complex [Cp*RhCl2]2 (Cp* = pentamethylcyclopentadiene) as the catalyst for this transformation. The reactions were performed in ethanol at room temperature.
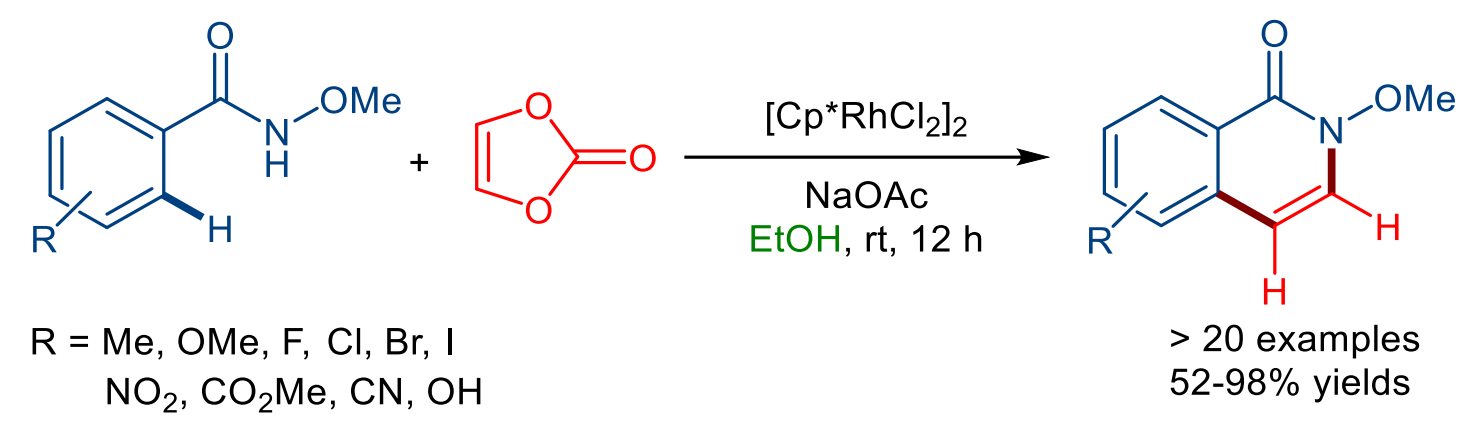
The developed method is environmentally friendly as it operates in biomass-derived ethanol under mild conditions and eliminates the need for stoichiometric external oxidants. The approach has a broad substrate scope and showed excellent regioselectivity. Further transformations of the obtained 3,4-unsubstituted isoquinolones can provide access to 4-substituted isoquinolones and 3,4-unsubstituted isoquinolines.
- Green Synthesis of 3,4‐Unsubstituted Isoquinolones through Rhodium(III)‐Catalyzed C‒H Activation and Annulation in Ethanol,
Vikash Kumar, Gandeepan Parthasarathy,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300914