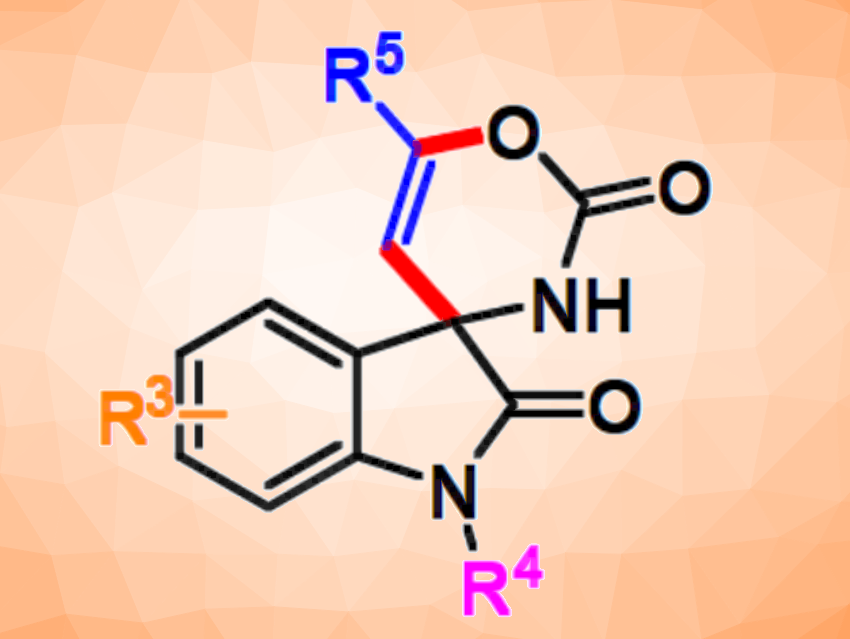
Functionalized spirooxindoles are found in different natural products and can be useful pharmaceutically active species. Thus, methods for the selective synthesis of spirooxindoles are interesting research targets.
Thomas Scattolin, Università degli studi di Padova, Italy, A. Stephen K. Hashmi, University of Heidelberg, Germany, and colleagues have developed a method for the gold-catalyzed synthesis of six-membered spirocarbamate oxindole derivatives (pictured) via a step-wise formal [4+2] cycloaddition of terminal alkynes or ynamides and isatin-derived ketimines. Isatin is a 2,3-diketo derivative of indole. The team also studied the in vitro antitumor activity of the products against breast cancer cells.
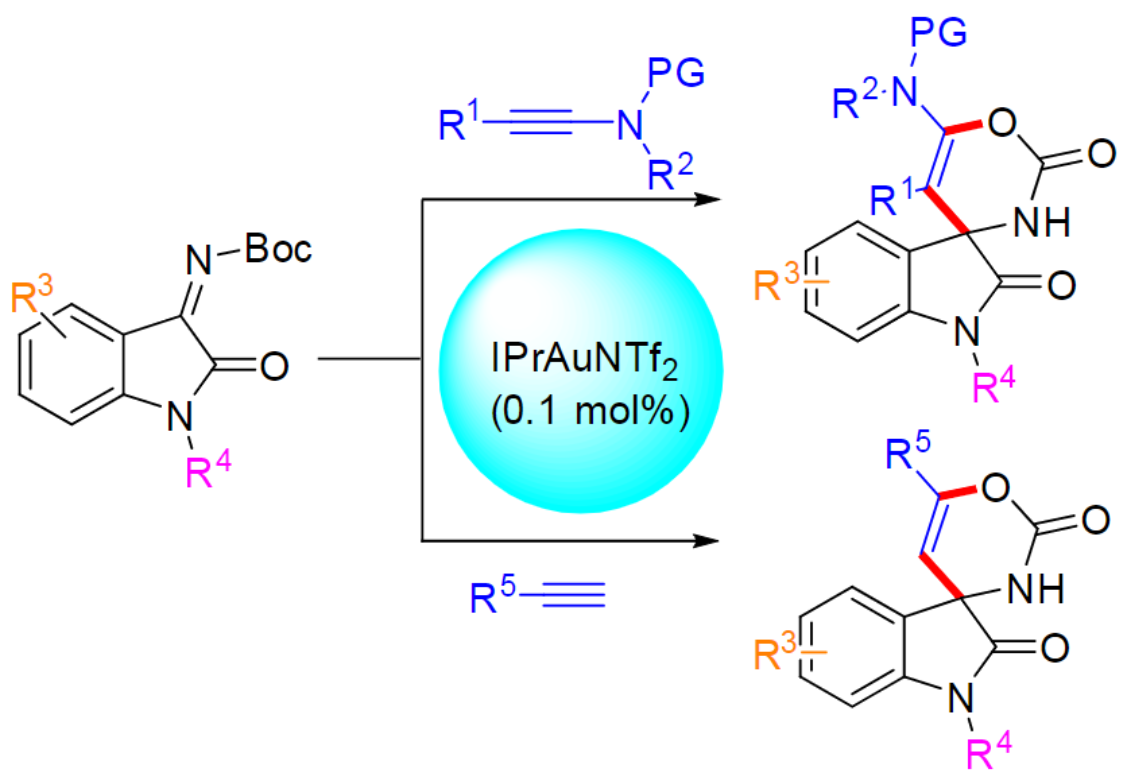
The researchers used IPrAuNTf2 as the catalyst (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, Tf = trifluoromethylsulfonyl) and dichloromethane (DCM) as the solvent. The reactions were performed at room temperature and the desired products were obtained in mostly high yields. A variety of functional groups were tolerated.
A preliminary screening of the antitumor activity of some of the products showed useful cytotoxicity. The screening allowed the team to observe structure-activity relationships that could be useful for further drug development.
- Gold‐Catalyzed Formal [4+2] Cycloaddition as Access to Antitumor‐Active Spirocyclic Oxindoles from Alkynes and Isatin‐Derived Ketimines,
Yaowen Liu, Martin C. Dietl, Robin Heckershoff, Chunyu Han, Hongwei Shi, Matthias Rudolph, Frank Rominger, Isabella Caligiuri, Kanwal Asif, Muhammad Adeel, Thomas Scattolin, A. Stephen K. Hashmi,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202304672