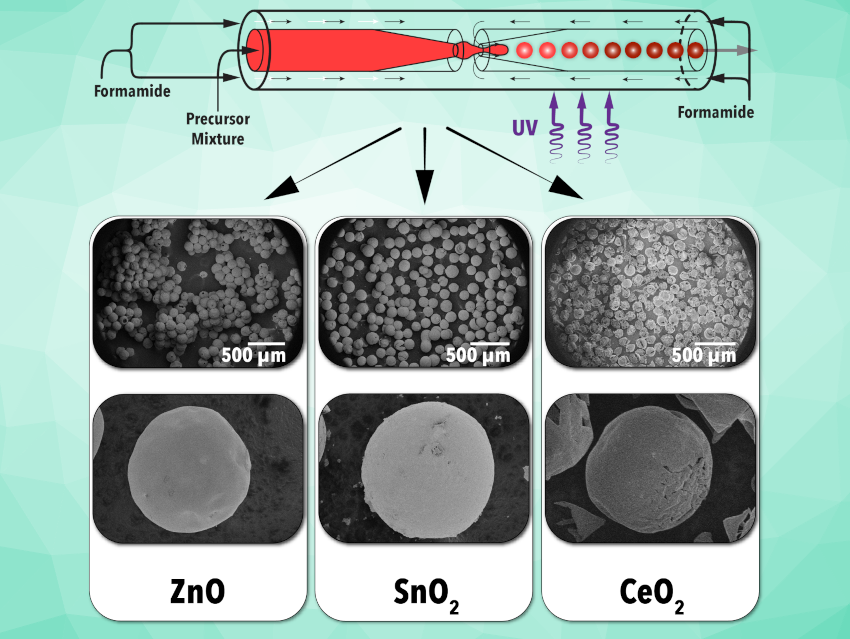
Metal oxides are important, broadly applicable classes of materials. They are used, for example, in the fields of catalysis, adsorption, or energy storage. Monodisperse metal oxide microparticles with sizes around 100 µm are particularly interesting in this context.
Milad Abolhasani, North Carolina State University, Raleigh, USA, and colleagues have reported a generalized flow synthesis technique, enabling the preparation of a variety of single and mixed-metal oxide microparticles using metal-organic precursors instead of the previously utilized metal alkoxides. The metal oxide microparticles were synthesized in flow using a flow-focusing microreactor (pictured at the top).
Microdroplets were formed from a nonpolar phase containing a metal-organic precursor, a photocurable polymer (ethoxylated trimethylolpropane triacrylate, ETPTA), toluene as the solvent, and a photoinitiator (2-hydroxy-2-methylpropiophenone). The metal oxide precursor was continuously fed into the microreactor through the inner channel of the flow-focusing microreactor, while a polar continuous phase (formamide) was continuously fed through the outer region, resulting in monodisperse droplets where both phases were forced through a constricted “exit”. The microdroplets were then exposed to ultraviolet light to cure the polymer. The resulting polymerized microparticles were dried and annealed to form metal oxide microspheres.
Utilizing this new synthesis technique, ZnO, SnO2, and CeO2 microparticles (pictured) were prepared. The particles were characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD), diffuse reflectance UV-Vis spectroscopy, and energy-dispersive X-ray spectroscopy (EDS). The generalizability of the flow synthesis technique was further demonstrated by synthesizing an exemplary mixed metal oxide (LaPrO3), leading to surface areas one order of magnitude larger than those achieved with traditional methods.
- Flow Synthesis of Single and Mixed Metal Oxides,
Zachary S. Campbell, Steven Baro, Yunfei Gao, Fanxing Li, Milad Abolhasani,
Chem. Methods 2022.
https://doi.org/10.1002/cmtd.202200007