Frustrated Lewis pair (FLP) catalysts can be useful alternatives to transition-metal catalysts. FLPs combine a Lewis acid and a Lewis base that cannot form an adduct due to steric hindrance, and some FLPs can activate small molecules. There also are examples of Intramolecular FLPs, in which the Lewis-acidic center and the Lewis-basic center are spatially constrained in the same molecule. In FLPs, group[nbp]13 and group 14 (tetrylene) elements can each behave with either Lewis-acidic or Lewis-basic character.
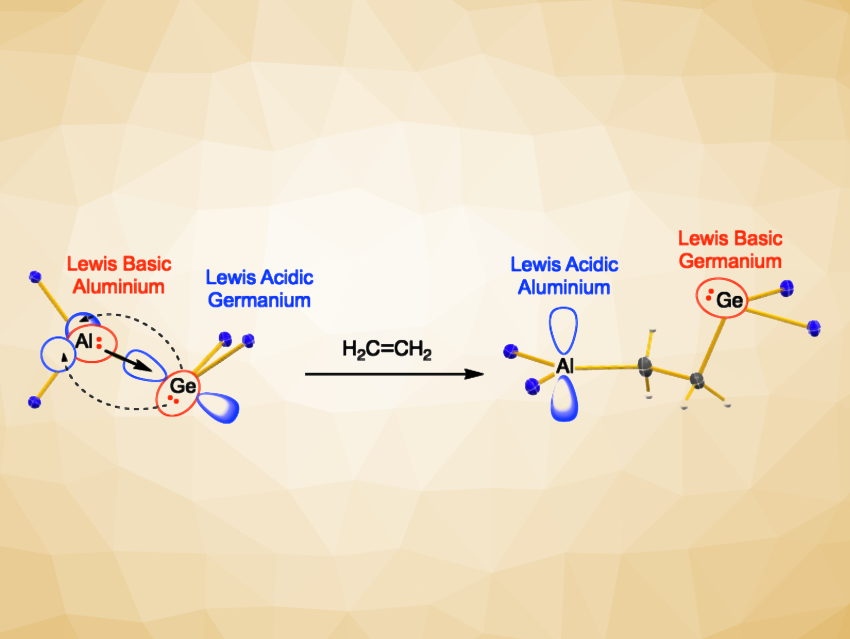
Claire L. McMullin, University of Bath, UK, J. Robin Fulton, Martyn P. Coles, Victoria University of Wellington, New Zealand, and colleagues have synthesized molecules that combine a low-valent aluminium unit with a tetrylene center, featuring an Al–E{14} bond (E{14} = Ge, Sn, Pb). The team found that these compounds can act as FLPs and activate small molecules (pictured). They reacted the potassium aluminyl compuund K[Al(NON)] ([NON]2– = [O(SiMe2NDipp)2]2–, Dipp = 2,6-iPr2C6H3) with group 14 chloroamidinates of the type E{14}(Am)Cl ([Am]– = [tBuC(NDipp)2]–. They obtained (NON)Al–E{14}(Am) Lewis pairs with unsupported Al–E{14} bonds, including the first structurally authenticated Al–Pb bond.
The products were characterized using, e.g., NMR, UV-Vis, and Mössbauer spectroscopy as well as X-ray diffraction, and their bonding situation was evaluated using quantum-chemical calculations. The team found evidence of an Al–E{14} σ-bond involving a Lewis-basic Al and a Lewis-acidic tetrylene, with back-donation from the E{14} element’s lone pair into an orbital on the Al center that is derived from s/p-orbitals. Reactivity studies showed that the Al–Ge compound can react with CO2 to form a dioxocarbene, while the Al–Sn compound forms a carbonate under the same conditions. Reactions of the Al–Ge or Al–Sn compounds with ethene give products with an ethylene bridge (example pictured), forming a new class of intramolecular FLPs with Lewis-acidic Al and Lewis-basic Ge/Sn centers.
- Frustrated Lewis Pairs from Al–E{14} Bonds (E{14} = Ge, Sn, Pb),
George W. A. Smith, Samuel E. Neale, Matthew J. Evans, Xuning Li, Junhu Wang, Michael G. Gardiner, Claire L. McMullin, J. Robin Fulton, Martyn P Coles,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202404206