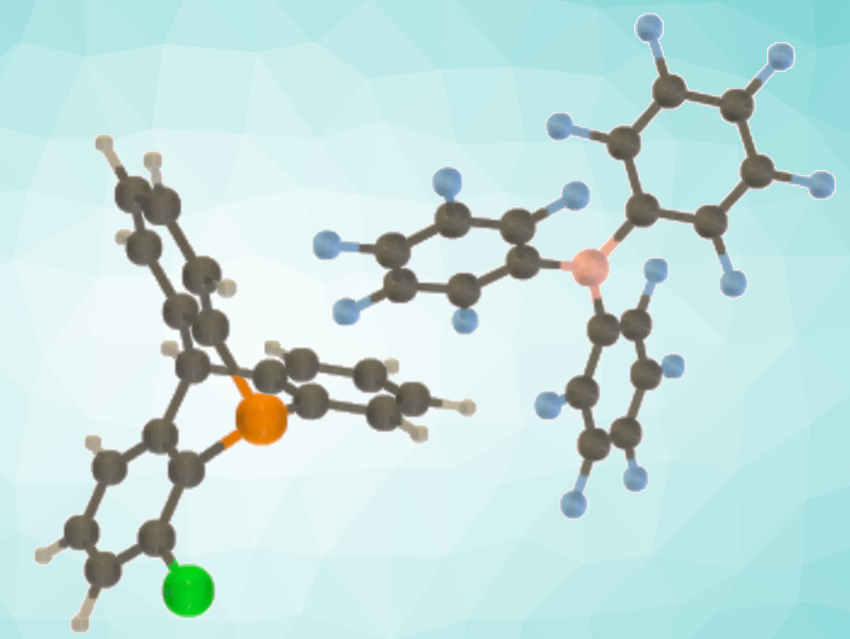
Frustrated Lewis pair (FLP) catalysts can be useful alternatives to transition-metal catalysts for hydrogenation reactions. FLPs combine a Lewis acid and a Lewis base that cannot form an adduct due to steric hindrance. Some FLPs can activate small molecules such as H2. The hydrogenation of unactivated olefins with H2 under FLP catalysis, however, remains a challenge.
Guillaume Berionni, University of Namur, Belgium, and colleagues have developed an FLP-catalyzed hydrogenation of unactivated alkenes with H2. The team used phosphatriptycene derivatives to design new FLPs (example pictured) that can tackle this challenging catalytic transformation. The reactions were performed directly with dihydrogen gas in a low-pressure reactor, using 1-chloro-9-phosphatriptycene together with tris(pentafluorophenyl)borane (B(C6F5)3) as the FLP catalyst. This approach allowed the hydrogenation of a series of unactivated alkenes including, e.g., cyclohexene, cyclopentene, and hex-1-ene.
The researchers attribute the unique reactivity of 9-phosphatriptycenes to their strained scaffold, which enables the generation of strongly acidic phosphonium ions after heterolytic dihydrogen cleavage. This allows a C=C bond protonation and drives the overall hydrogenation of unreactive alkenes, which was confirmed by mechanistic investigations and quantum chemical calculations. According to the researchers, this work represents the first example of an FLP-catalyzed hydrogenation of unactivated alkenes.
- Frustrated Lewis Pair Catalyzed Hydrogenation of Unactivated Alkenes With Sterically Hindered 9‐Phosphatriptycenes,
Damien Mahaut, Benoît Champagne, Guillaume Berionni,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200294