Frustrated Lewis pair (FLP) catalysts can be useful alternatives to transition-metal catalysts for hydrogenation reactions. FLPs combine a Lewis acid and a Lewis base that cannot form an adduct due to steric hindrance. Some FLPs can activate small molecules such as H2, which makes them useful in catalysis. Immobilizing FLPs on a solid support material is useful to obtain catalysts that are easy to separate and recycle. Using metal nanoclusters, which can be useful catalysts themselves, as a support for FLP catalysts is an interesting research target.
Fengyu Li, Hui Shen, Inner Mongolia University, Hohhot, China, Nanfeng Zheng, Xiamen University, China, and Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, and colleagues have immobilized 2-methoxybenzenethiol on copper nanoclusters to form a solid catalyst with FLP-type active sites that can promote regioselective hydrogenation processes. The team prepared the functionalized nanoclusters from the copper salt Cu(MeCN)4BF4, 2-methoxybenzenethiol, and triphenylphosphine as an additional ligand, using NaBH4 as a reducing agent.
The resulting products are atomically precise nanoclusters of the type [Cu34S7(RS)18(PPh3)4]2+ (RSH = 2-methoxybenzenethiol). The thiolate groups of the 2-methoxybenzenethiol molecules are bound to the copper nanocluster and help to stabilize it. The Lewis-basic OMe groups and Lewis-acidic copper atoms on the cluster surface together form the frustrated Lewis pair.
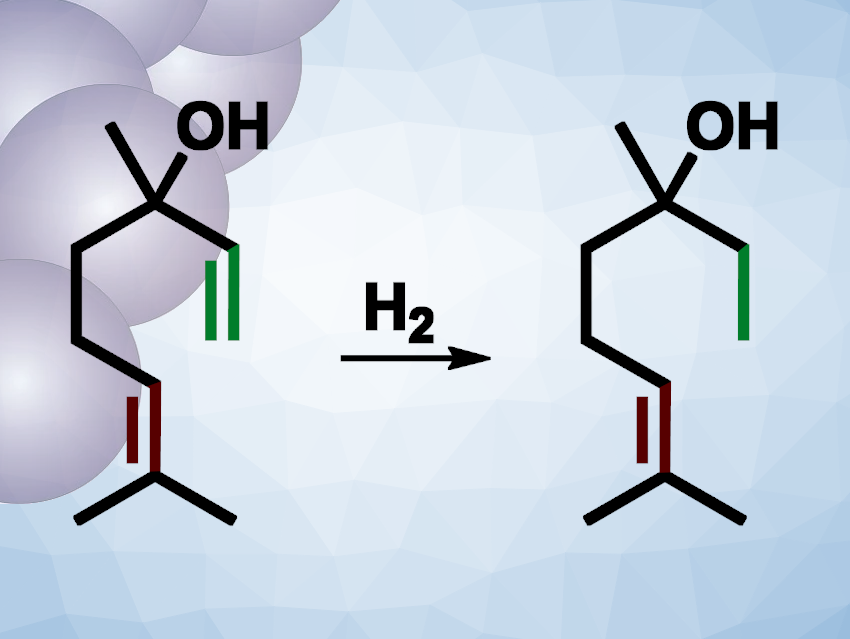
The team proposes that the steric crowding stemming from the ligands should favor the selective hydrogenation of less sterically crowded substrates or sites. They used linalool as a model substrate (pictured) and found that under 50 bar of H2 at 30 °C, hydrogenation at the less crowded site (pictured in green) proceeded with 100 % selectivity and very high conversion. The conversion remained high for several cycles of reusing the catalyst. According to the researchers, their nanocluster-based FLP activates the hydrogen via a heterolytic pathway, which improves the effectiveness of the desired transformation under ambient conditions.
- Anchoring Frustrated Lewis Pair Active Sites on Copper Nanoclusters for Regioselective Hydrogenation,
Simin Li, Qingyuan Wu, Xuexin You, Xiaofei Ren, Peilin Du, Fengyu Li, Nanfeng Zheng, Hui Shen,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c10251