The structural and electronic properties of aluminum–boron-bonded species are interesting, but their synthesis has proven to be challenging. Alumaboranes (R2Al–BR2), for example, are compounds containing two adjacent Lewis-acidic centers and generally require stabilization, e.g., by the coordination of a Lewis base or the formation of a three-center-two-electron bond. Similarly, adducts with Al(I) donors and an Al→B dative bond are rare.
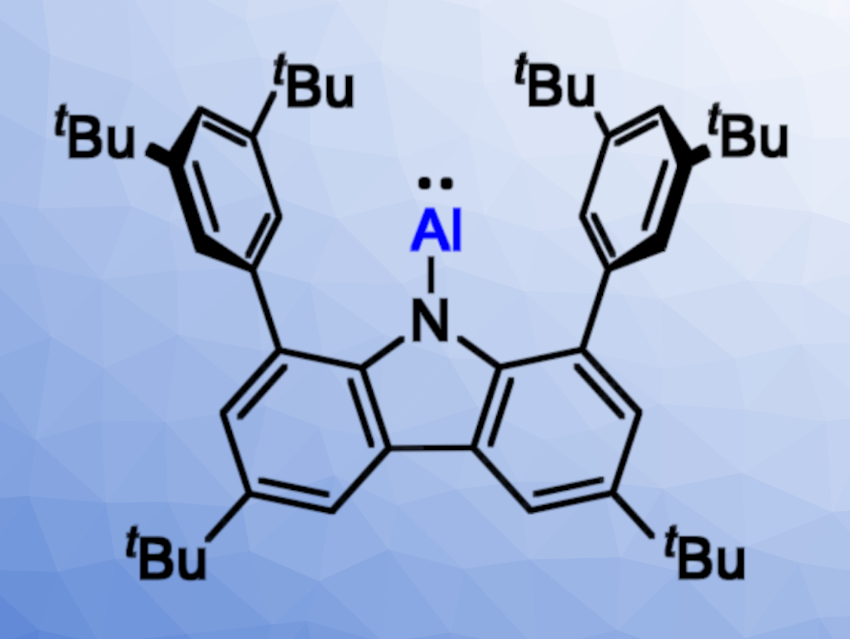
Liu Leo Liu and Xin Zhang, Southern University of Science and Technology, Shenzhen, China, have investigated the reactivity of a free aluminylene towards boron Lewis acids and synthesized an alumaborane as well as Lewis adducts with an Al→B dative bond. The researchers used the free aluminylene [N]-Al ([N] = 1,8-bis(3,5-di-tert-butylphenyl)-3,6-di-tert-butylcarbazolyl, pictured above).
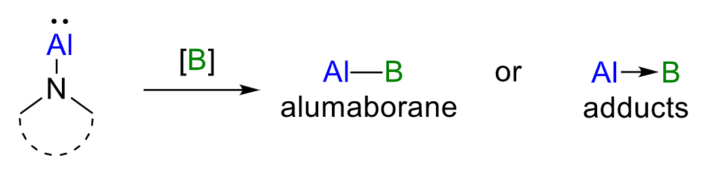
The team was able to prepare a rare alumaborane in 43 % yield by reacting [N]-Al with tetraphenyldiboroxane (Ph2BOBPh2). In addition, they prepared adducts with an Al→B dative bond by reacting [N]-Al with tris(pentafluorophenyl)borane (B(C6F5)3) or bis(pentafluorophenyl)borane (HB(C6F5)2).
X-ray crystallography was used to confirm the structures of the products, and quantum chemical computations were used to investigate their electronic structures. The team discovered that the formation of the alumaborane occurred through a concerted oxidation addition reaction at the aluminylene center. These findings contribute to the understanding of the fundamental properties of aluminum–boron-bonded species.
- Reactivity of a Free Aluminylene towards Boron Lewis Acids: Accessing Aluminum‐Boron‐Bonded Species,
Xin Zhang, Liu Leo Liu,
Eur. J. Inorg. Chem. 2023.
https://doi.org/10.1002/ejic.202300157