Fluorinated organic compounds can be useful, e.g., in pharmaceutical chemistry or materials science. One path to partially fluorinated molecules is the defluorination of perfluoroalkyl compounds. However, this type of transformation can be challenging to achieve and require harsh reaction conditions due to the strength of C–F bonds. The development of new methods for perfluoroalkylation followed by defluorination is, thus, an interesting research target.
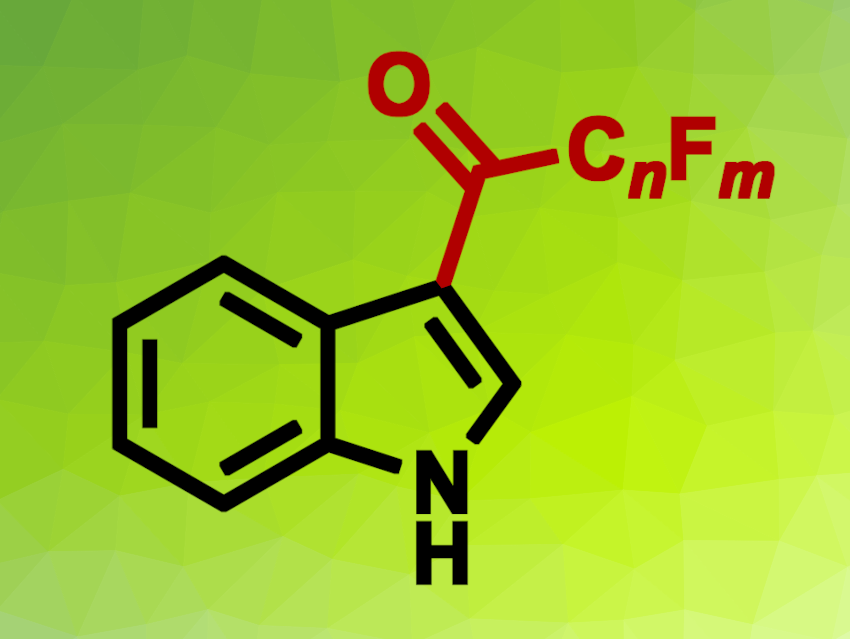
Faqiang Leng, Capital Medical University, Beijing, China, and colleagues have developed a method for a one-pot perfluoroalkylation/defluorination reaction of indoles, which results in an overall fluoroacylation (simplified product structure pictured). The team reacted indole derivatives with perfluoroalkyl iodides in the presence of Na2S2O4 using a mixture of dimethyl sulfoxide (DMSO), acetonitrile, and water as the solvent. The reactions were performed at 100 °C.
The desired fluoroacetylated products were obtained in yields of up to 80 %. Electron-donating substituents led to higher yields and electron-withdrawing groups to lower yields. The reaction could be used to introduce –CO–C3F7, –CO–C2F5, or–CO–CF3 substituents to the indole substrates. The researchers propose a reaction mechanism that involves the transformation of the perfluoroalkyl iodide into a perfluoroalkanesulfinate (CnFmSO2Na), which was coupled with the indole to give a perfluoroalkylated intermediate. This intermediate is then defluorinated under acidic reaction conditions via a carbocation species. Overall, the work provides access to useful indole derivatives under mild reaction conditions.
- Direct Fluoroacylation of Indole with Perfluoroalkyl Iodides,
Guizhao Wang, Nianhua Yu, Ying Wen, Faqiang Leng,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02148


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
