Fluoride anions are indispensable in biology. It is important for the proper growth and development of nails, hair, teeth, and bones, and also helps in preventing dental caries. Fluoride concentrations above 1.5 ppm are hazardous and can lead to fluorosis, bone disorder, disrupt the immune system, and can even lead to death.
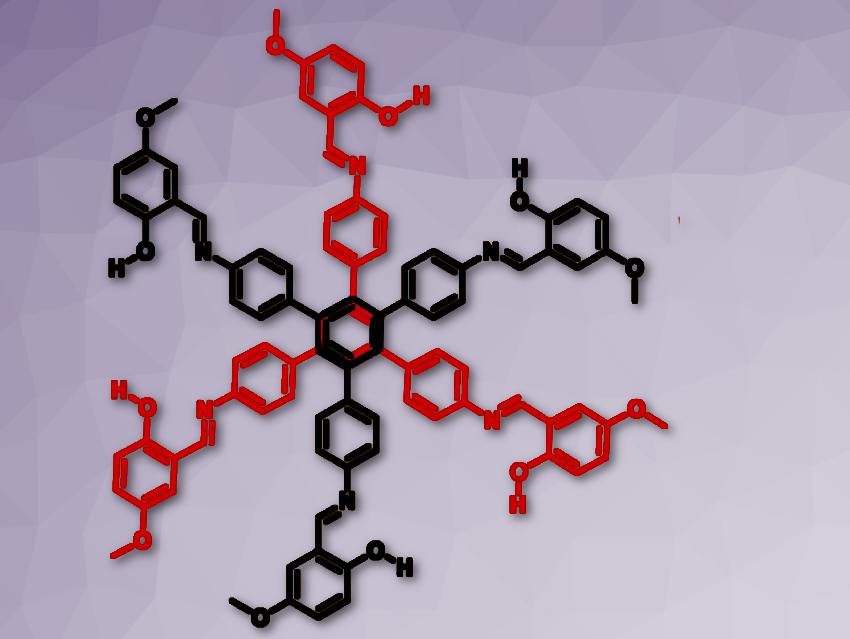
Prakash P. Neelakandan and colleagues, Institute of Nano Science and Technology, Mohali, India, have developed a chemosensor that can detect the presence of fluoride ions through visual fluorescence changes. A tripodal molecule was synthesized incorporating three salicylaldimine moieties that formed a dimer structure resembling a six-petal flower in the presence of fluoride ions (pictured above and below).
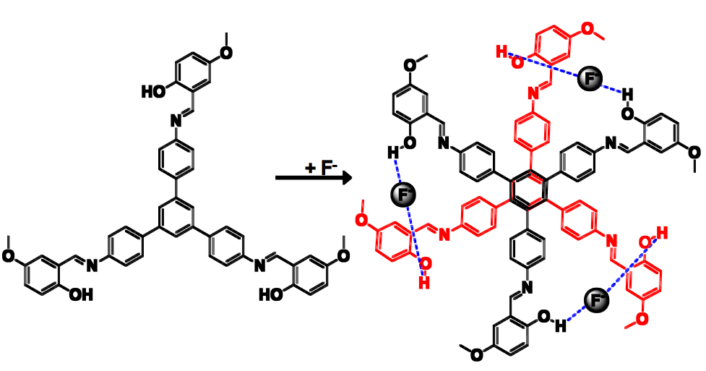
The fluoride functions as a bridge between the molecules (see picture above). Dimer formation further led to aggregation which resulted in intramolecular rigidification thereby leading to changes in fluorescence that could be detected by the naked eye.
This molecule was observed to selectively detect fluoride under a variety of conditions such as in solution, thin film, and under cellular environment with a detection limit in the ppm regime.
- Aggregation‐Induced Emission, Mechanofluorochromism, and Selective Fluoride Detection by a Tripodal Salicylaldimine,
Parvati Marandi, Nidhi Tyagi, Prakash P Neelakandan,
ChemPlusChem 2022.
https://doi.org/10.1002/cplu.202100555