In order to deliver cancer therapeutics to tumor cells, the cells’ membranes must be overcome. Juan Chen, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada, Gang Zheng, Princess Margaret Cancer Centre and University of Toronto, and colleagues have discovered a simple way to achieve this using lipid nanoparticles containing EDTA (ethylenediaminetetraacetic acid). An unusual mechanism of action of EDTA is responsible for this useful effect.
Intracellular Drug Delivery
One of the greatest challenges in cancer therapy is the targeted delivery of drugs, contained in nanoparticles, to tumor cells, where they can then kill the cells. Unfortunately, cell membranes are highly selective in terms of what they allow to pass. Receptors on the membrane surface usually act as gatekeepers, but devising the precise molecular key to unlock them is tricky.
Liposomes offer one alternative: These nanoscale lipid spheres simply fuse with the cell membrane, transporting their therapeutic payloads into the cell in the process. However, more efficient intracellular uptake is still sought to increase drug efficacy.
A “Cell Opener”
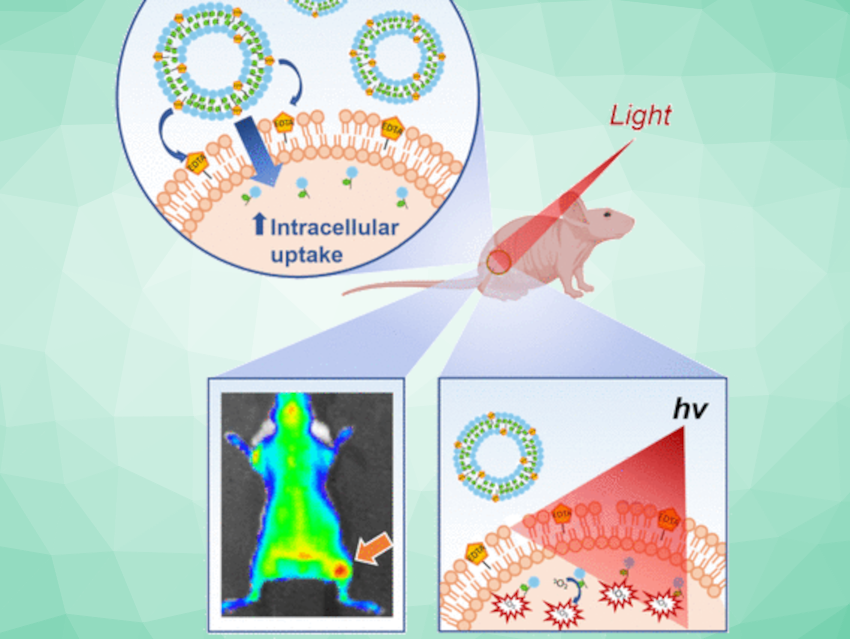
The team took a closer look at EDTA as a new way to gain entry to cancer cells. EDTA can chelate metal ions, in other words securely bind them and remove them from their environment. In biomedicine, EDTA is used to treat heavy metal poisoning, and it is used as a contrast agent for bioimaging.
The researchers hypothesized that the chelating effect might be useful as a cell opener too. As Zheng explains, “A high concentration of EDTA is known to permeabilize bacterial membranes by chelating metals (e.g., calcium ions) on their membranes. We wondered whether incorporating an EDTA-lipid conjugate into liposomes could enhance their intracellular uptake at a much lower EDTA concentration that is non-toxic to human cells.”
Enhanced Uptake
The team, therefore, incorporated an EDTA-lipid compound into drug-loaded liposomes, in order to deliver them into tumor cells. The experimental results exceeded their expectations, showing drastically improved internalization in both cell lines and in a mouse model. In tumor-infected mice, the drug—porphyrins used for photodynamic therapy—caused the tumor to decline and improved the survival rates of the mice significantly.
However, to the team’s great surprise, the chelate effect had nothing to do with the enhanced internalization. “This process is totally independent of its metal chelation properties,” says Zheng of the team’s findings. Instead, the group discovered that EDTA acted like a detergent to change the characteristics of the cell membrane, making it more fluid and flexible to promote nanoparticle uptake. The group now plans to apply this newly discovered mechanism to a general EDTA-lipid-based strategy for cells to absorb liposomal nanoparticles for enhanced therapy.
- Novel Strategy to Drive the Intracellular Uptake of Lipid Nanoparticles for Photodynamic Therapy,
Tiffany Ho, Keegan Guidolin, Ali Makky, Michael Valic, Lili Ding, Jiachuan Bu, Mark Zheng, Miffy H. Y. Cheng, Jeremy Yau, Juan Chen, Gang Zheng,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202218218