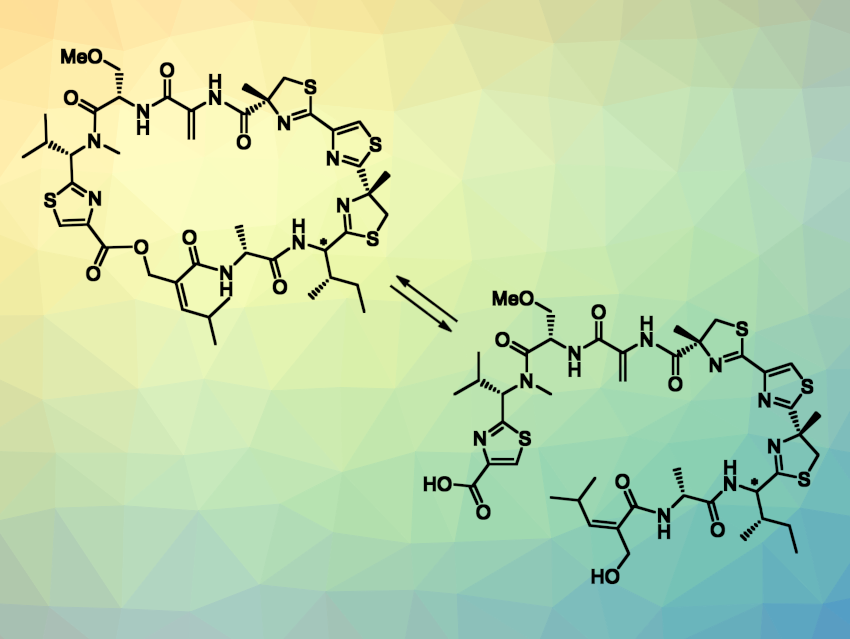
The thiamyxins are a group of thiazole/thiazoline-rich natural products that were isolated from myxobacteria. Thiazole and thiazoline-containing natural products can have useful biological activities, and thiamyxins show interesting activity against RNA viruses. Thiamyxins A and B are cyclic depsipeptides (pictured above on the left) that differ only in the configuration at one stereocenter. Thiamyxins C and E (pictured above on the right) are open-ring variants of thiamyxin B and A.
Uli Kazmaier, Saarland University, Saarbrücken, Germany, and colleagues have performed the first total synthesis of thiamyxins A–C, as well as thiamyxin E, which had been undescribed so far. The team first prepared several building blocks: a thiazoline–thiazole–thiazoline fragment, protected peptides, and a Z-alkenoic acid. These building blocks were coupled, and the resulting intermediate underwent a saponification/elimination step to give a mixture of the diastereomers thiamyxin E (main product) and C (minor product). The team attempted to purify the compounds using preparative HPLC, but the diastereomers were inseparable and further isomerized to a 1:1 mixture due to a labile stereocenter.
Next, a macrolactonization of the thiamyxin C/E mixture was performed to obtain the cyclic variants thiamyxins A and B. In this case, purification by preparative HPLC gave thiamyxins A and B as pure diastereomers. Thus, the cyclic derivatives are less sensitive to epimerization compared to the open-chain variants. After the separation, the pure cyclic thiamyxins could be used to obtain the open-chain variants separately: Saponification of thiamyxin A or B using lithium hydroxide gave thiamyxin E or C with a diastereomeric ratio of 92:8 and 95:5, respectively. Overall, the work provides access to four natural products via the same route.
- Total Synthesis of Thiamyxins A–C and Thiamyxin E, a Potent Class of RNA-Virus-Inhibiting (Cyclo)depsipeptides,
Kevin Bauer, Uli Kazmaier,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202305445