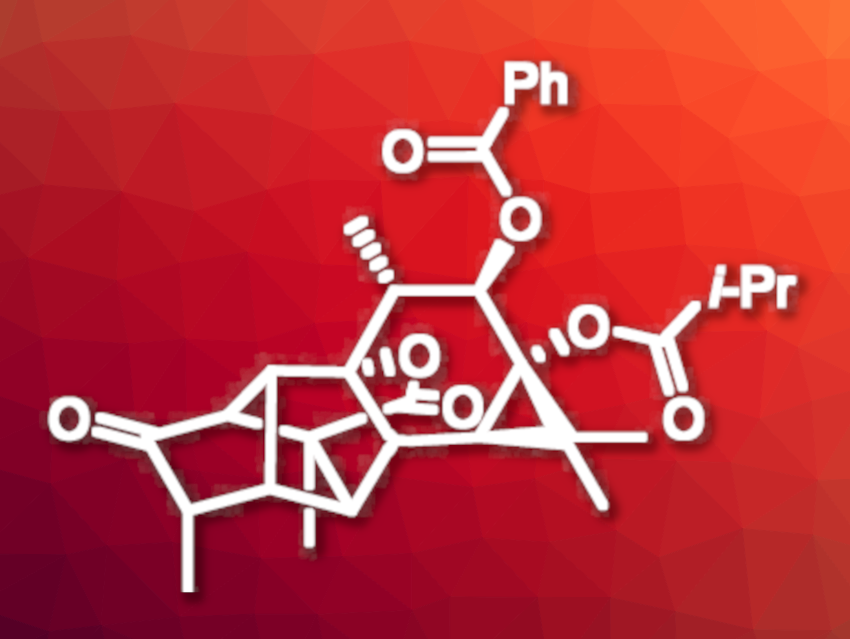
(+)-Pedrolide (pictured) is a natural product that was first isolated from Euphorbia pedroi plants (a species of flowering plant in the spurge family). The compound is a diterpenoid with interesting bioactivity. It has a complex 5–5–6–6–3-fused pentacyclic carbon skeleton, which makes it an interesting and challenging target for total synthesis.
Marlene Fadel and Erick M. Carreira, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, have performed the first total synthesis of (+)-pedrolide. The team first prepared a cyclohexanone derivative from 4-methoxyphenol. This intermediate was converted to a precursor for a key Diels–Alder reaction. In the team’s approach to this key step, norbornadiene serves as a surrogate for cyclopentadiene via a Diels–Alder reaction cascade. They obtained an intermediate with a bicyclo[2.2.1]heptene core. The researchers then finalized the synthesis using an epoxidation/epoxide opening sequence to install an alcohol, which was converted to the desired ketone group, followed by deprotection, acylation, and benzoylation steps.
The desired product was obtained in 20 steps. The spectroscopic data of the product was in agreement with that of the isolated natural product. According to the researchers, their approach broadens the scope of the Diels–Alder reaction for congested, synthetically challenging compounds.
- Enantioselective Total Synthesis of (+)-Pedrolide,
Marlene Fadel, Erick M. Carreira,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c02113