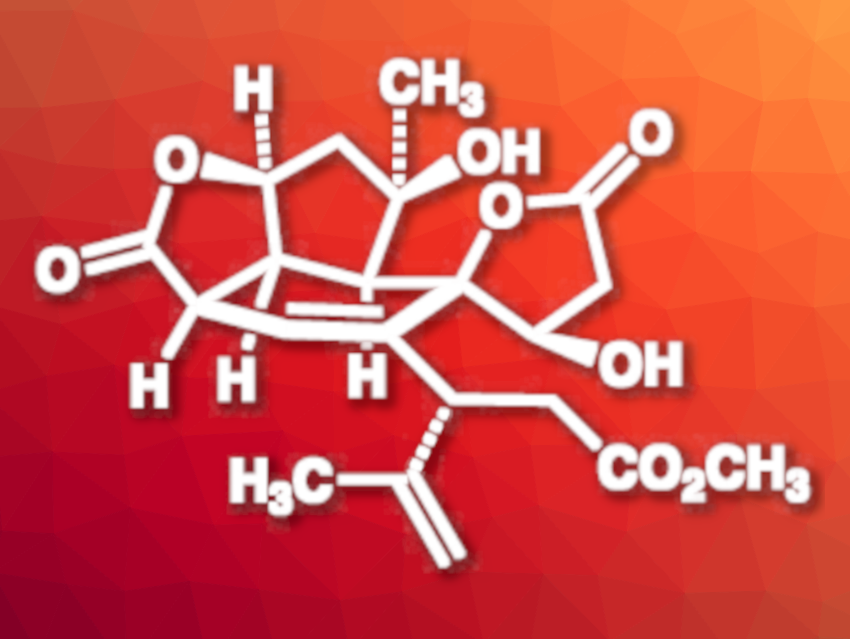
Havellockate (pictured) is a natural product that was isolated from Sinularia granosa corals. It has a complex polycyclic structure, featuring a highly oxygenated cis-fused tricyclic core and a spiro-fused β-hydroxybutanolide ring. The compound has eight stereogenic centers, seven of which are contiguous. This structure makes havellockate a challenging target for total synthesis, and no completed syntheses of the compound had been reported so far.
Brian M. Stoltz, California Institute of Technology, Pasadena, USA, and colleagues have performed the first total synthesis of havellockate. The team used a convergent strategy: They first prepared an aldehyde and a sulfone building block, which were connected using a Julia–Kocienski olefination. The resulting intermediate was subjected to an acylation/Diels–Alder reaction sequence to create the [5–5–6] framework of the product. This was followed by a three-step oxidation protocol and the construction of the spiro-fused ring using an allylzinc reagent generated from 3-bromo-1-acetoxypropene and a copper-catalyzed aerobic oxidation.
The structure of the product was confirmed using X-ray crystallography as well as NMR and infrared (IR) spectroscopy. The team found that the optical rotation of their product did not match the reported value for the natural material. Since there is no available sample of the product, they could not determine whether the natural product could be enantiomeric to the synthesized product or whether there was an error in the original measurements. According to the researchers, this can be addressed when a new sample of the natural product becomes available.
- Asymmetric Total Synthesis of Havellockate,
Nicholas J. Hafeman, Melinda Chan, Tyler J. Fulton, Eric J. Alexy, Steven A. Loskot, Scott. C. Virgil, Brian M. Stoltz,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c09583