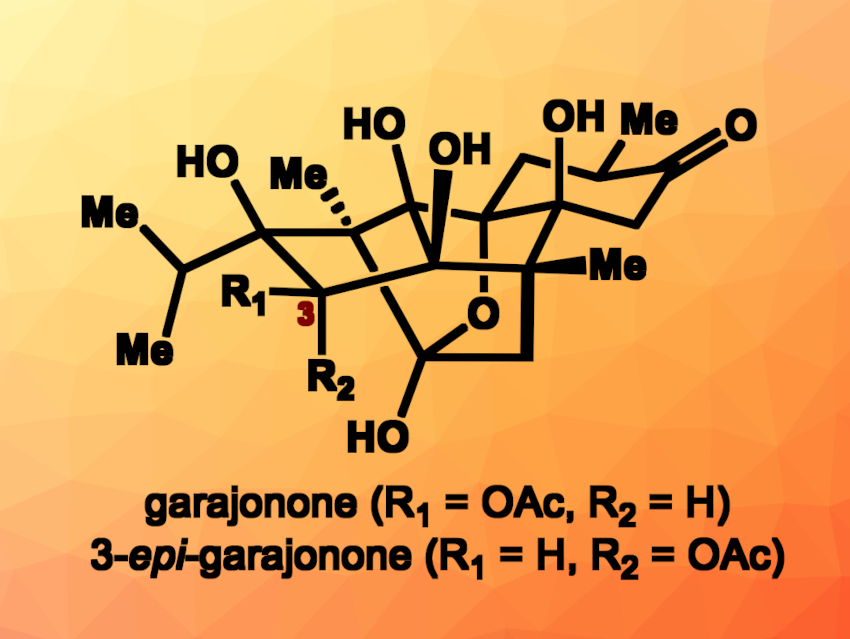
Ryanodine is a diterpene natural product first isolated from a shrub that is native to Central and South America. Ryanodine analogs can show interesting bioactivities, but can be challenging to synthesize due to their complex structures. Garajonone (pictured), for example, is a ryanodine diterpenoid isolated from Persea indica, an evergreen in the laurel family that is native to the Canary Islands. It has a polycyclic, cage-like framework with a hemiketal unit and features a high density of oxygenated, tetrasubstituted carbon centers. Garajonone was initially incorrectly identified as an epimer, i.e., 3-epi-garajonone (pictured). Both garajonone and 3-epi-garajonone are difficult targets for total synthesis.
Yu-Ming Zhao, Shaanxi Normal University, Xi’an, China, and colleagues have performed the first chemical syntheses of garajonone and its epimer 3-epi-garajonone. The team used a two-stage synthetic strategy inspired by the two stages of the biosynthesis of polycyclic diterpenes: first a cyclization stage, followed by an oxidative stage. The researchers started by preparing an aryl lithium reagent, which was coupled with a cyclic enone to give a bicyclic intermediate. After the oxidation of an alcohol group to restore an enone functionality, a palladium-catalyzed cyclization was used to close a third ring.
The resulting tricyclic intermediate underwent several redox steps and a lactonization to give an epoxide-functionalized, tetracyclic intermediate. Starting with the hydrogenative opening of the epoxide, this intermediate was then further converted to construct the hemiketal unit of the product. To complete the synthesis, an acetate group was introduced at C3, and protecting groups were removed.
Using this strategy, the team obtained the desired garajonone. They found that the spectral properties of the product were in good agreement with those of the natural product. Using much of the same path, the researchers also prepared 3-epi-garajonone. They hope that thedeveloped synthetic pathway could also provide access to other ryanodine diterpenoids and their analogues and allow studies of their biological activities.
- Total Synthesis of Ryanodane Diterpenoids Garajonone and 3‐epi‐Garajonone,
Jin-Bao Qiao, Long Meng, Jia-Yi Pei, Hui Shao, Yu-Ming Zhao,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202417647