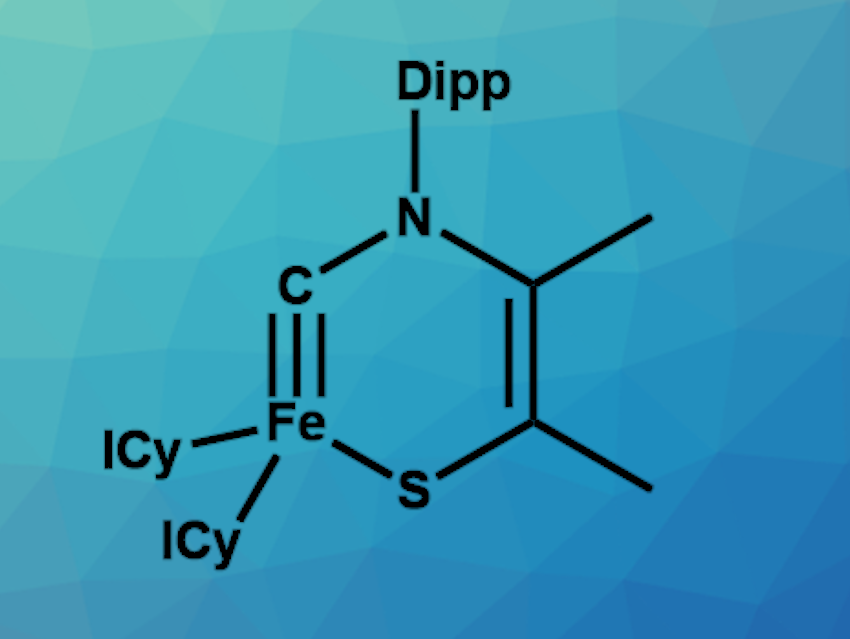
Transition metal carbyne complexes are compounds with a triple bond between carbon and a transition metal (LnM≡CR). They have uses, e.g., in catalysis or materials science. Tuning their electronic structure—in particular, their spin state—for different applications could be useful. However, the strong field of carbyne ligands generally leads to complexes with low-spin ground states (singlet or doublet). So far, there had been no examples of isolable metal terminal carbyne complexes in high-spin states (triplet or above).
Jun Zhu, Xiamen University, China, and The Chinese University of Hong Kong, Shenzhen, Guangdong, China, Liang Deng, Shanghai Institute of Organic Chemistry and Hangzhou Institute for Advanced Study, University of the Chinese Academy of Sciences, and colleagues have synthesized the first triplet metal terminal carbyne complex, the cyclic iron carbyne complex [(ICy)2Fe(κ2–C,S-CN(Dipp)CMeCMeS)] (pictured, ICy = 1,3-bis-cyclohexyl-imidazol-2-ylidene, Dipp = 2,6-diisopropylphenyl). The team reacted the three-coordinate iron(0) N-heterocyclic carbene complex [(ICy)2Fe(η2-CH2═CHSiMe3)] with a thiazol-2-ylidene in diethyl ether at room temperature.
The triplet iron carbyne complex was obtained via oxidative addition in a yield of 76 %. It was characterized using single-crystal X-ray diffraction, 57Fe Mössbauer spectroscopy, magnetic susceptibility measurements, and density functional theory (DFT) studies. The data confirmed that the complex has a triplet ground state and a comparatively weak Fe≡C bond. It can undergo carbyne coupling reactions and addition reactions with electrophiles.
- A Triplet Iron Carbyne Complex,
Jiahao Rao, Shicheng Dong, Chengbo Yang, Qing Liu, Xuebing Leng, Dongyang Wang, Jun Zhu, Liang Deng,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c09280