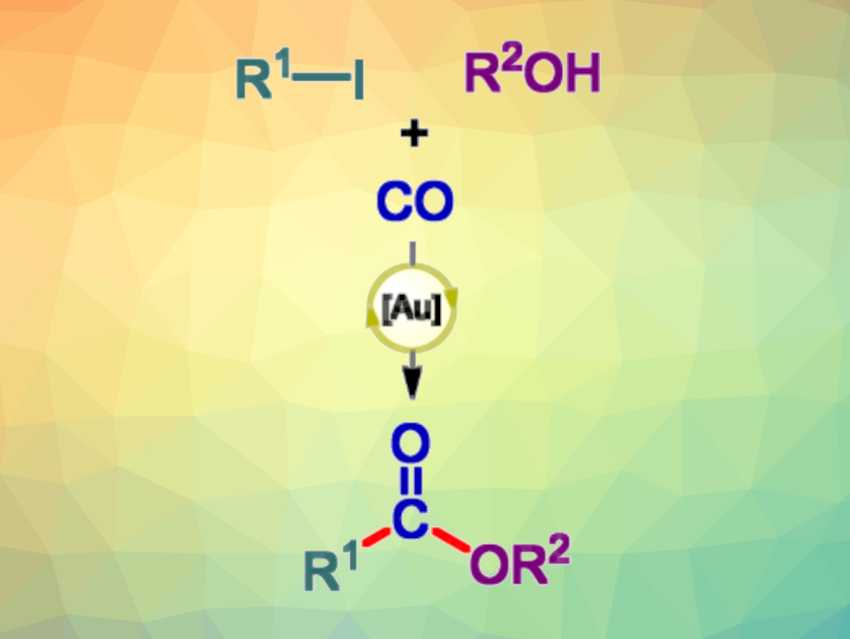
Using low-cost, easily available carbon building blocks is interesting in organic synthesis. Carbon monoxide, for example, can serve as a cheap C1 synthon. It can be used in carbonylation reactions to give compounds such as aldehydes, ketones, carboxylic acids, or esters. This type of reaction can be catalyzed by transition metals, and gold catalysis has become an interesting research field. However, there had been no reported examples of carbonylations of aryl and vinyl iodides in gold chemistry so far.
Vincent Gandon, Paris-Saclay University, Orsay, France, Nitin T. Patil, Indian Institute of Science Education and Research, Bhopal, India, and colleagues have developed the first example of a gold-catalyzed alkoxy-carbonylation of aryl and vinyl iodides to give esters (general reaction pictured). The team’s approach is based on Au(I)/Au(III) redox catalysis, using a gold chloro complex with a di(1-adamantyl)-2-dimethylaminophenylphosphine ligand that binds via the P and N atoms. Using this complex as the catalyst, silver triflate (AgOTf) as an additive, and dichloroethane (DCE) as the solvent, the team reacted a variety of iodo(hetero)arenes and vinyl iodides with different alcohols and CO (1 atm) at 70 °C.
Under these conditions, the desired esters were obtained in mostly good to excellent yields. The researchers found that the transformation shows high selectivity for aryl and vinyl iodides over fluorides, chlorides, bromides, etc. They propose that the reaction proceeds via an attack of the alcohol reaction partner on an intermediate carbonyl-gold(III) complex, followed by reductive elimination.
- Gold‐Catalyzed Alkoxy‐Carbonylation of Aryl and Vinyl Iodides,
Vivek W. Bhoyare, Asish Bera, Vincent Gandon, Nitin T. Patil,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202410794