The development of energetic materials is a useful research field, e.g., for the aerospace industry and civil engineering purposes. Energetic materials usually contain fuel and oxidizer in the same compound. For example, in highly nitrated hydrocarbons, the organic backbones serve as fuel and the nitro groups serve as oxidizers. Optimizing the properties of such compounds (energy, density, and oxygen balance) while retaining good safety can be challenging. Azoles can be useful starting points with good thermal stabilities and multiple sites for substitution.
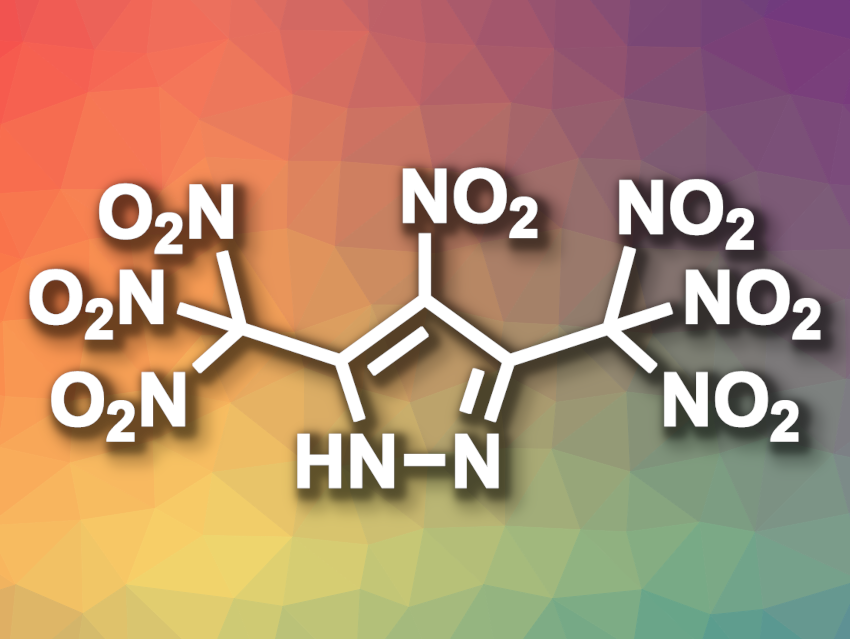
Searching for new energetic materials, Jean’ne M. Shreeve, University of Idaho, Moscow, USA, and colleagues have synthesized the first azole molecule bonded to seven nitro groups, 4-nitro-3,5-bis(trinitromethyl)-1H-pyrazole (pictured). The team obtained the target compound via the stepwise nitration of 3,5-dimethyl-1H-pyrazole. The first nitration was performed using H2SO4 and KNO3 at 110 °C, giving 3,5-dimethyl-4-nitro-1H-pyrazole. The methyl groups were converted to chloroximes, followed by a second nitration step using trifluoroacetic anhydride and HNO3 at 0 °C and a reaction with KI to obtain dipotassium 3,5-bis(dinitromethyl)-4-nitro-1H-pyrazole. The target product is obtained by a third nitration step using H2SO4 (98 %) and HNO3 (100 %).
The highly nitrated azole was characterized using single-crystal X-ray diffraction. It has a high density and a positive oxygen balance and was found to be sensitive toward impact and friction. The thermal decomposition of the product starts at 131 °C. According to the team, the detonation properties are comparable to those of the traditional benchmark explosive RDX.
- Manipulating nitration and stabilization to achieve high energy,
Jatinder Singh, Richard J. Staples, Jean’ne M. Shreeve,
Sci. Adv. 2023.
https://doi.org/10.1126/sciadv.adk3754