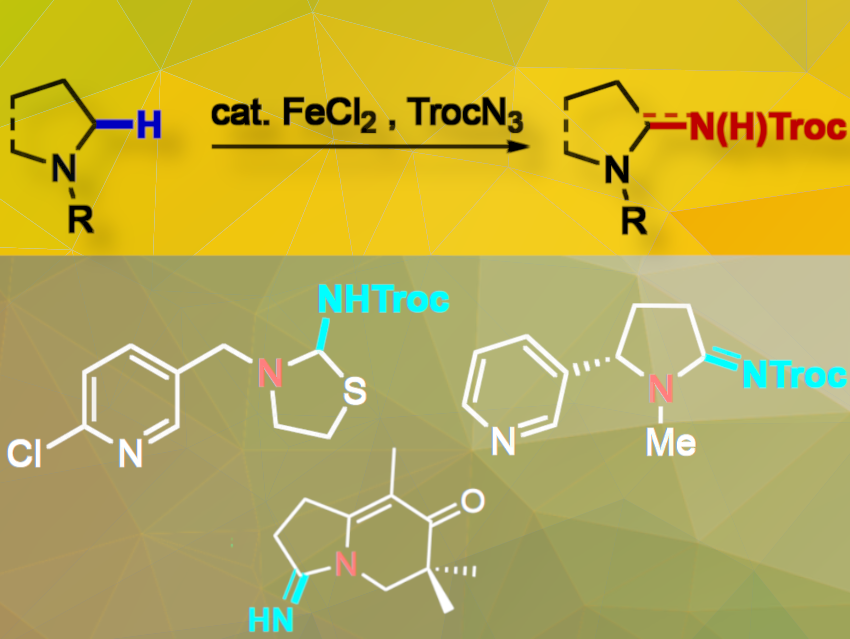
Nitrogen-heterocycles are important in drugs and natural products; more than 80 % of approved small molecule drugs contain an N-heterocycle, with pyridines, piperidines, and pyrrolidines being the most represented. C–H amination is a simple way to create C–N bonds, but most methods use expensive metal catalysts.
Andrea Geraci and Olivier Baudoin, University of Basel, Switzerland, have developed a practical method for the site-selective intermolecular C(sp3)–H amination of N-heterocycles using inexpensive FeCl2 as a catalyst. The catalyst reacts with Troc-azide (Troc = 2,2,2-trichloroethoxycarbonyl) to form a reactive Fe-nitrenoid, producing N2 as the sole byproduct. The proposed reaction mechanism involves a turnover-limiting generation of an Fe(III)-imidyl radical, followed by homolytic C–H bond cleavage of the N-heterocyclic substrate and fast radical rebound. The C–H amination occurs site-selectively at the α-position to the nitrogen atom, even when weaker C–H bonds are present.
The reaction produces aminals or amidines, which are protected by the Troc group. The process is compatible with a wide range of amines, including pharmaceutically relevant compounds and complex molecules, and is compatible with late-stage functionalization tactics.
To demonstrate the synthetic potential of the method, the team achieved a two-step synthesis of (–)-cotinine from (–)-nicotine. They also synthesized the reported structure of senobtusin, an atypical alkaloid isolated from Senecio obtusatus. Several species belonging to the genus Senecio have been used in traditional Chinese medicine due to their richness in bioactive pyrrolizidine alkaloids, flavonoids, and sesquiterpenoids. Supposedly the molecule contains an amidine group. Although the compound was not highly stable, the team fully characterized it. However, its NMR data did not align with that reported by the isolation team, showing substantial discrepancies throughout the molecular framework. The molecule is depicted above at the very bottom.
- Fe-Catalyzed α-C(sp3)–H Amination of N-Heterocycles,
Andrea Geraci, Olivier Baudoin,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202417414