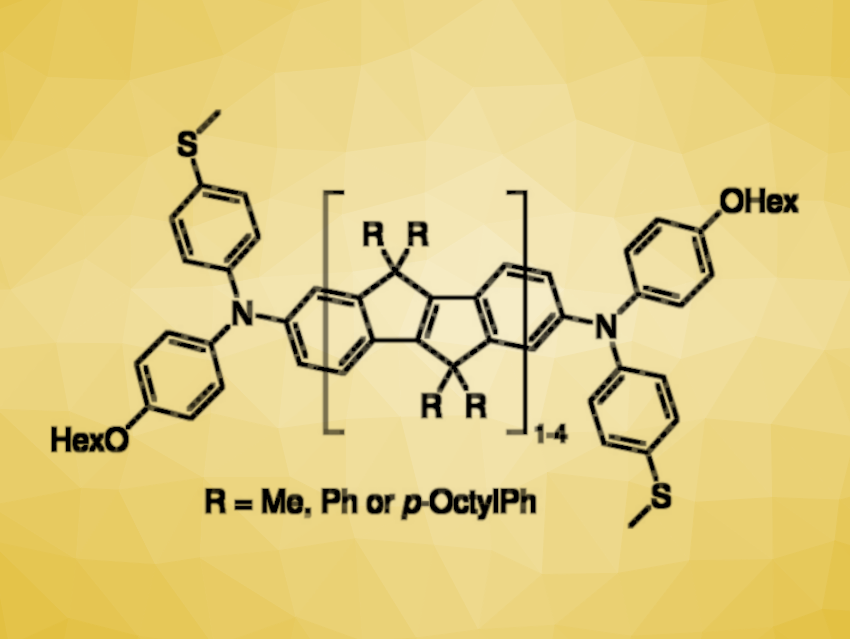
Molecular electronics could allow researchers to make integrated circuits as small as possible. For this, molecule-sized electronic components such as conductive molecular “wires” are needed. Usually, there is an exponential conductance decay with increasing length for such molecular wires. This can, however, be circumvented by the introduction of unpaired spins at the ends of the molecules, which allow for high conductance if the spins are within range for electronic coupling.
Gemma C. Solomon, University of Copenhagen, Denmark, Rainer F. Winter, University of Konstanz, Germany, and colleagues have developed a class of molecular wires (general structure pictured) with lengths up to 3.6 nm and exceptionally high conductance. These properties were achieved by combining readily oxidizable diarylamine termini, which allow the introduction of unpaired spins at the ends of the molecule, with a rigid, planarized core structure based on carbon-bridged oligo(phenylene-vinylene)s that improves delocalization. The team obtained the singly and doubly oxidized forms of the molecular wires via oxidation with silver hexafluoroantimonate.
Thiomethyl groups were used as anchors to bind the wires to gold atoms on the surface of nanoelectrodes, allowing the researchers to measure the molecular conductance of the nanowires using a scanning tunneling microscope break junction (STM-BJ) technique. They found that the oxidized forms show excellent conductance, with a conductance increase of up to 1,800[times] compared with the corresponding neutral compounds. Overall, the work expands the selection of long and exceptionally conductive molecular wires.
- High Molecular Conductance and Inverted Conductance Decay over 3 nm in Aminium-Terminated Carbon-Bridged Oligophenylene-Vinylenes,
Luisa K. I. Rieger, Susanne Leitherer, William Bro-Jo̷rgensen, Gemma C. Solomon, Rainer F. Winter,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c13901