Tim Lüddecke is the Group Leader of the Animal Venomics group of the newly established Department for Bioresources of the Fraunhofer Institute for Molecular Biology and Applied Ecology Gießen, Germany. Here, he talks to Vera Koester for ChemistryViews about his passion for and extensive knowledge of venomous animals, the search for new proteins, applications as insecticides or in medicine, and the challenges of the German academic system.
You research animal venoms. What is the proper term for scientists who study this field?
Toxicologists study how toxins distribute in the body and how they work. Toxinologists study natural toxins and the animals or organisms that produce them. It is a subcategory of toxicology.
I’ve read that it was your childhood dream to become a researcher in animal venoms. Why was that?
I don’t know, I probably was just a very strange kid. Since kindergarten, I’ve always had a fascination with toxic animals, mostly snakes but also spiders and scorpions.
I come from a small village, and whenever I was out with friends to play soccer or somewhere at the sports ground, I would usually skip the soccer and instead venture through the bushes to search for spiders, simply because I preferred spiders over anything else. I even declared to my mom that when I grew up, I would become the most important venom researcher on Earth … in all my modesty.
Hey, and you are on a good track to be just that …
She then thought I was crazy but here we are. I don’t know, I’ve been obsessed ever since.
So you were never afraid of spiders and scorpions, obviously?
No, I used to bring all kinds of animals with me in my pockets and keep them in glasses and aquariums at home. As I grew older, around the age of 13 or 14, I started breeding scorpions, and then I added spiders to the mix. I was breeding hundreds of tarantulas in my room, and I’ve never returned to a normal life since.
Wow, I would have been afraid to enter that room.
Most people are initially afraid, but once they get used to it and learn more about spiders, they often start to like them. That’s very funny. Especially with the big hairy ones, people are super afraid in the beginning, but when I then tell them that my spiders have names like Agate, Fräulein Müller, and so on, they usually find it funny. And when they came over more often, they become accustomed to it and sometimes even develop a liking for them. My wife, for example, who used to be arachnophobic, now owns a couple of baby tarantulas and takes care of them.
So maybe this is the effect: The better you know something, the more you like it?
Yes, I think that’s why most people are afraid of these creatures because they are not familiar with them. It’s not because they’re actually dangerous; snakes are, but spiders and scorpions are usually not. In most people’s perception, everything is dangerous, including centipedes, scorpions, and spiders, but they’re not.
Do you still have any pets at home?
I have many. Currently, my wife and I live with 50 tarantulas, 12 scorpions, ten leeches, three snakes, and a horse. However, the horse lives outside.
Which animals do you study?
Our emphasis is on arachnids, so spiders, scorpions, and pseudoscorpions, but we also started a research line on insect venoms last year. We mainly work with hymenopterans there, which are bees, wasps, and ants, and now we just got funding from the German Research Foundation (DFG) to start a project on snake venoms as well. I love snakes, it’s my hobby project, so to say.
So, in totality, we actually study all types of venomous creatures. That’s a major difference from most working groups. They typically focus on a narrow taxonomic area, such as studying snakes or corn snails. However, we study animals across taxonomic borders on a broader scale.
How do you choose the animals you study?
The mission of my team is to explore uncharted territories, places where no one else has been looking so far. We focus on studying organisms that are often overlooked.
When you consider global venom research, most colleagues study species that are medically significant because the major driver behind venom research is to find ways to improve treatments. However, the reality is that 99 % of all venomous animals are not dangerous. Take, for example, small ants. In addition, they are very difficult to study because they’re so small. As a result, they are understudied because it takes a lot of work, it’s difficult, and there’s no urgent medical need.
However, we believe that when you look into the neglected species, you are more likely to find novel biomolecules that you can use. The challenge is that these groups are super diverse. For instance, there are approximately 50,000 species of spiders. So it is difficult to choose which species to study. So we also include a lot of taxonomy and phylogeny [a diagram that depicts the lines of evolutionary descent of different species from a common ancestor] in our research. We examine the evolutionary relationships between species and analyze the phylogenetic tree to identify the lineages with the least amount of research done. By strategically focusing on these less-explored areas, we aim to uncover new insights.
Occasionally, I also select species based on personal preference, such as the snake project I mentioned. It’s my hobby project, specifically focused on our native Kreuzotter; Common European Viper in English.
Where do you get the venoms or the animals from?
The sources are quite diverse. For most species found in Germany, we do fieldwork to collect them. That’s what we do with most of the spiders and ants. In fact, the ants for our project were collected here on the university campus.
In addition, I cooperate with zoos and private people as we have a huge community in Germany that breeds venomous animals, mostly snakes but also spiders. Zoos are important as they house very rare species that require specialized keepers. A good example here is a venom project on lizard venom we are currently working on. I collected venom from Komodo dragons at the Leipzig Zoo. This was super cool because normally you would not be able to collect Komodo dragon venom.
Is it because there are very few of these animals left?
No, there are several Komodo dragons. They are endemic to Komodo Island. However, visiting Komodo Island itself is quite difficult. The entrance fee for Komodo National Park is quite high, and there are additional costs for flights and logistics. Also, it is super remote and thus it’s difficult to get there in the first place and also to get the samples out of Komodo. It’s super-hot and you work with proteins, so you need to cool them all the time.
Komodo dragons can grow up to three meters, so they are too large for private keepers. There are very few zoos that keep them, and we rely on their cooperation to obtain the venom.
Do you collect the samples, or do zoos deliver them to you?
Most of the time, I collect the samples myself. Sometimes, we cooperate with people who know the animals and how to collect the venom. In many cases, we first need to figure out ways to collect the venom.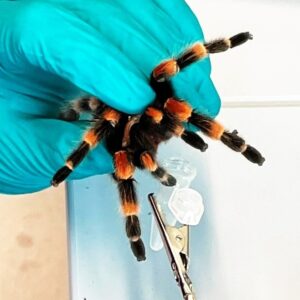

Collecting venom from snakes is relatively easy: You grab the snake behind its head, milk it by letting it bite through a membrane, and collect the venom. This is a common practice in snake farms. Collecting venom from venomous lizards is also quite simple: You need to grab the lizard and put a pipette in its mouth to get the saliva. However, when you work with small arthropods, you need to find alternative methods to collect the venom.
For tarantulas, we first anesthetize the spider. Then we use a small pincher, a pair of tweezers with an attached circuit to provide electroshocks to the spider, which initiates venom release. This method doesn’t always work as some spiders are a bit reluctant to release venom, perhaps because they’re very gentle. In such cases, I stop to avoid harming the spider and proceed with a more aggressive animal.
For smaller spiders, this method can sometimes be effective, but often you need to kill the spider and dissect the venom glands to extract the venom chemically. Similarly, with bees; you must kill them to obtain the venom as their stinger is removed when a bee stings.
We have developed a super cool method for collecting ant venom. You put an ant in a tube filled with ethanol. The ant gets dizzy and agitated. It panics and starts stinging into the solution. We then remove the ant and put it in a small box where it recovers. This is repeated around 200 times, and then we evaporate the ethanol to obtain the venom.
What do you then do with the venom? You said you’re focusing on the proteins.
Once we have the venom, the next step is to disentangle which molecules are in there. We have two options for this: proteomics or transcriptomics. In proteomics, we use mass spectrometry to analyze the components of the venom. In transcriptomics, we extract mRNA from the venom gland and sequence the transcripts. If possible, we use both methods.
Proteomics can only discover components that are already known and present in databases. In taxonomic lineages where no toxins have been described, we are likely to find new components that are not in the database. In such cases, you need to create your own database using transcriptomics and then verify the presence of the transcripts on the protein level using proteomics. However, this is a dream scenario. Very often, only one of the methods works.
If we can combine the results, we get a holistic overview of the venom transcript, the components, and then, we can recreate the venom composition using computers. From there, we select interesting molecules for further study. This is done mostly by bioinformatics, and, to be honest, sometimes just by gut feeling.
Our criteria vary depending on what we are looking for in a given venom. For example, we may find molecules in the venom that are similar to known molecules with promising activities against microbes. This is one of our main research areas. We may also discover components with similarities to anti-cancer agents, which also become candidates for further investigation.
On the other hand, we may come across completely novel proteins with no known similarity. Of course, we want to study those as well, but that can be challenging. We cannot isolate them chemically because the venom yield is so low. In such cases, we need to genetically modify E. coli strains to produce the proteins in the lab, which is difficult as we don’t know anything about the folding and modifications of the proteins.
For components with similarity to known proteins, the process is often much easier. In some cases, we can even synthesize them chemically because they are very simple. We have already published some studies on the activity of such molecules and found some very cool potential drug candidates.
Does that mean you have a huge team of people to cover all of these different tasks?
Actually, no. My team is a junior research group. It was established in 2018 as a combinatorial group between the Fraunhofer Institute and the University of Gießen. In 2021, it was merged into one project group under the roof of Fraunhofer under my leadership. We started with two people, just me and my postdoc, who is currently on maternity leave. Since then, we grew to a total of eight people. Sometimes we have students here, so we have a few more hands to work with, but since students require more supervision, it doesn’t actually help too much.
The key here is that we are a team of different experts. I am a toxinologist and an animal guy, the other postdoc is a straight-up technology person, so she’s really into protein expression. My Ph.D. students come from various backgrounds, including bioengineering, basic biology, and agriculture, and we also have technicians. In addition, we cooperate with other Fraunhofer institutes and other research institutes, especially in Australia where the big experts are located.
Why is it that in Australia? Is it because they have most of the toxic animals?
When you look at venom research, you will find that the community is very heavily concentrated in Australia. That’s because they have most of the extremely toxic species. Consequently, in the last century, the Australians fostered venom research due to medical needs. Australia also had the financial resources to support this research, unlike other regions such as Southeast Asia or Africa, which faced similar needs but lack the economic means to support venom research to the required extent.
So in Australia venom research was supported by the government very early on, leading to its growth and the attraction of top researchers in the field.
You said that the proteins can be used in cancer research, drug development, or against microbes. What are you focusing on?
We have multiple focal areas in my group. One of my personal favorites is insecticides. I believe that we need novel ways to combat pest insects to sustain the global food supply. Currently, the insecticides being used seem to drive the insect decline. Therefore, we need to replace these with better alternatives. In my opinion, spiders and scorpions are the best insect killers in the world, so we want to learn from them how this is done best.
In the realm of medicine, our focus is primarily on pathogens. When we started our research group, we already had several working groups dedicated to antibiotic research and antivirals in the institute. We started right in the Covid-19 pandemic, which taught us that we don’t have effective treatments for viral diseases. Therefore, we are in the process of establishing a biosecurity level 3 lab, which will enable us to work with dangerous viral pathogens. Additionally, we cooperate with colleagues in Frankfurt who do pharmacological screenings. We supply them with our components for testing anti-cancer activity and painkilling properties. The actual research in these fields is mostly done by our Frankfurt colleagues, while we provide them with the necessary components.
Personally, I would like to expand our research to include neurological diseases, such as pain. Currently, I don’t know how and if this is possible at all, but I believe there is also a big market for developing treatments for some psychiatric diseases. Many diseases, such as depression, are linked to ion channel mutations, and many spider venoms can modulate ion channels. Exploring this avenue could lead to the development of novel antidepressants. According to my knowledge, no one has ever done that so far.
Furthermore, we have just started a completely new research line focused on spider venom enzymes. Industrial goods production is a very important contributor to climate change, and species decline due to pollution, largely due to synthetic processes. We aim to replace some of these synthetic processes with more sustainable, greener alternatives using enzymes. Especially spider venoms contain many potent and very resistant enzymes, as they have evolved to function outside the spider’s body.
Spider inject their venom into their prey, and the enzymes in the venom are active in another organism, which is quite unique. Usually, enzymes have a narrow optimum, and left and right of that they don’t work anymore. There is a huge potential to learn more about how to make better enzymes for industrial applications by looking into spider venom enzymes. So far, this area of research has not been extensively explored, but we now have a Ph.D. student starting to investigate it.
In summary, our research covers a wide range of areas, including pesticides, medicine, and industrial applications. The direction we focus on more strongly in the future will depend on upcoming developments and funding availability.
When do you think we will see products, such as in agriculture or pesticides, on the market?
Well, pesticides are already available on the market. There is an American company called Vestaron that was founded based on a peptide from Australian funnel web spiders. They have already launched other products, I believe, three years ago. I had the opportunity to speak to one of the founders of the company at the World Congress of Toxinology in Abu Dhabi last autumn, and he told me that they are planning to release more products soon. So, the products are on their way. They are already being used in the American markets, but only hold a small percentage of the European market share.
I have been approached by several players in the insecticide industry to initiate collaborations, and we actually started one last year—however, I cannot talk about that in more detail. I believe there is a big market potential for these products. This is also my favorite project because I think making our society more sustainable is the key challenge we face. It’s not just about finding drugs because there will always be diseases that we cannot cure. Even if we find a drug for one disease, another will prevail, and ultimately, we all have to die at some point. However, studying venoms and working towards a more sustainable society is where we can really make a difference.
The same principle applies to the enzymes we are researching, but this field is still in its early stages, and I don’t have a clear idea of how it will develop.
What are your hopes for your research in the near future?
I’m actively looking for partners to increase the financial support for my group. We currently have funding secured until the end of 2024, with some projects receiving funding until 2026. However, my goal is to have a financial backup that would allow me to dedicate five to ten years of intense work to a single project, without the constant need to seek new funding every year. The short-term funding cycles make it difficult to plan and execute long-term projects. For example, developing a new technology typically requires a time frame of around ten years, which is not feasible within the constraints of two-year funding cycles.
On a personal level, I want to focus on creating an archive of native spider venoms. In Germany, we have 40 families of spiders, and my aim is to analyze the venom composition of each, at least with one representative member. This would provide us with a holistic understanding of the venoms of our native fauna so that we at least have an idea of what we can use as resources in the future.
Once this archive is established, potential industry partners interested in developing new products can approach us, and together we can design projects and use this archive to generate novel bioresources. However, this is a long-term project that would likely span many years. I would need to have staff for this and also the security to do it because I can only do this if the financing situation of my group is clarified for a longer period of time.
So the main challenge is to get enough funding?
Not the amount, as such. We have been quite successful in getting funding. But most funding programs typically run between two and four years, which is too short for an infrastructure-intense project that is not yet established. And if we want to collaborate with the industry for economic purposes, much of the research we are doing cannot be published due to the need to protect intellectual property. This makes it unsuitable for Ph.D. students who are required to publish their work. Moreover, the long time frame of the project makes it unsuitable for completion by a single Ph.D. or postdoc. These are the biggest problems that need to be addressed.
Your small team is extremely interdisciplinary, as you mentioned.
Yeah, that’s what makes it fun, and it’s also what makes it difficult sometimes. Communication becomes crucial when you have a group that includes bioinformaticians, zoologists, evolutionary biologists, bioengineers, and myself, the venom guy who is always thinking about toxic creatures and has some unconventional ideas. During lab meetings, it can be difficult to effectively communicate due to the diverse backgrounds and perspectives.
It’s challenging for my Ph.D. students because growing up in this environment requires them to learn everything simultaneously. I currently have three Ph.D. students who are all in the first six months of their program. I have exposed them to various papers, concepts, and methods right from the start, which can be overwhelming. However, this approach is beneficial because it allows them to develop a broad expertise compared to other Ph.D. students. While it may be difficult initially, it will at some point become a competitive advantage for them.
Any tips for someone who wants to start in your group or the field of animal venoms?
In venom research, people are generally friendly, but it’s considered a specialized field—Orchideenfach in German. If you want to have a career in this area, you’ll need to be very resilient because it can be challenging to find universities or institutions that focus on this field.
When you come to my group for a potential project, everything will be fine as long as there’s an open position or you come with your own funding. However, once you complete your project, you should be aware that you’ll be entering a competitive world because your expertise doesn’t fit into traditional academic disciplines like zoology or bioengineering. This is something I experienced firsthand when I was looking for positions after completing my Ph.D. It was pretty hard to find suitable opportunities.
I would say the most important qualities for someone interested in venom research, particularly in my group, are curiosity and motivation. It can be a challenging journey because things often don’t work initially. Each project we undertake goes onto untouched grounds. Unlike other groups that focus on a narrow field for many years, everything we do is completely new. None of these projects have been done before. The first year or two, it will feel like nothing is working, and it can be really crushing and depressing. However, in the end, things eventually will be fine.
Carrying optimism through extended difficult times is not easy.
Thank you for the fascinating insight into the world of toxinologists and animal venom research.
Tim Lüddecke, born in 1989, completed his training as a chemical laboratory technician in 2012. From 2012 to 2018, he studied biology with a specialization in biochemistry at the Technical University of Braunschweig, Germany. He completed his Ph.D. in 2021 at the Fraunhofer Institute for Molecular Biology and Applied Ecology IME in Gießen, Germany. Since 2018, he has also been an Associated Scientist at the LOEWE Center for Translational Biodiversity Genomics in Frankfurt am Main, Germany. Additionally, he has been the second chairman of the German Arachnological Society since 2019 and one of the representatives for Germany in the Management Committee (Substitute) of the EU COST Action “European Venom Network” since 2020.
As of 2021, Tim Lüddecke is the Group Leader of the Animal Venomics Department of the newly established Fraunhofer Institute for Bioresources in Gießen.
Selected Awards
- 2021: Prize for the best doctoral dissertation of the Justus Liebig University of Gießen, Germany
- 2023: Alumnus of the 72nd Lindau Nobel Laureate Meeting
Selected Publications
- T. Lüddecke, A. Paas, R. J. Harris, L. Talmann, K. N. Kirchhoff, A. Billion, K. Hardes, A. Steinbrink, D. Gerlach, B. G. Fry, A. Vilcinskas, Venom biotechnology: casting light on natures deadliest innovations using synthetic biology, Frontiers in Bioengineering and Biotechnology 2023, 11, 1166601. https://doi.org/10.3389/fbioe.2023.1166601
- S. Hurka, K. Brinkrolf, R. Özbek, F. Förster, A. Billion, J. Heep, T. Timm, G. Lochnit, A. Vilcinskas, T. Lüddecke. Venomics of the Central European Myrmicine Ants Myrmica rubra and Myrmica ruginodis, Toxins 2022, 14(5), 358. https://doi.org/10.3390/toxins14050358
- T. Lüddecke, V. Herzig, B. M. von Reumont, A. Vilcinskas, The biology and evolution of spider venoms, Biological Reviews 2022, 97, 163-178. https://doi.org/10.1111/brv.12793
- B. M. von Reumont, T. Lüddecke, T. Timm, G. Lochnit, A. Vilcinskas, J. von Döhren, M. Nilsson, Proteo-Transcriptomic Analysis Identifies Potential Novel Toxins Secreted by the Predatory, Prey-Piercing Ribbon Worm Amphiporus lactifloreus, Marine Drugs 2020, 18(8), 407. https://doi.org/10.3390/md18080407
- T. Lüddecke, B. M. von Reumont, F. Förster, A. Billion, T. Timm, G. Lochnit, A. Vilcinskas, S. Lemke, An Economic Dilemma Between Molecular Weapon Systems May Explain an Arachno-atypical Venom in Wasp Spiders (Argiope bruennichi), Biomolecules 2020, 10, 978. https://doi.org/10.3390/biom10070978
Also of Interest

Venomous animals and their poisons


