Xavi Ribas, University of Girona, Spain, and colleagues have explored how nickel(II) complexes can catalyze the attachment of trifluoro- and difluoroethoxy groups to C(sp2)-H bonds in aromatic compounds using a macrocyclic substrate. The unique stability of the substrate allowed the team to detect key intermediates and provided insights into the redox mechanism of the reaction.
Please describe what you did.
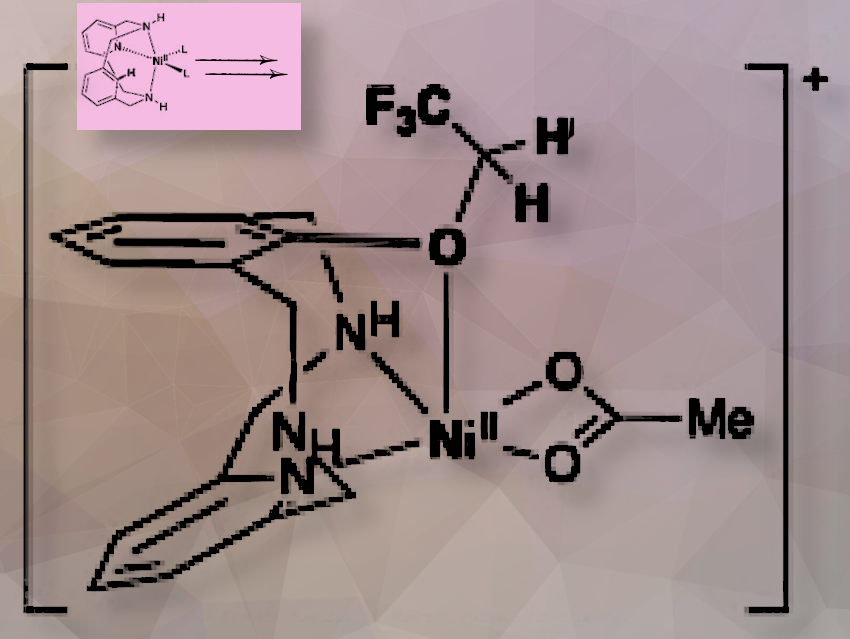
In this work, we describe the trifluoro- and difluoroethoxylation of C(sp2)-H bonds using common solvents such as trifluoroethanol (TFE) and difluoroethanol (DFE), mediated by nickel(II) complexes incorporating a model macrocyclic arene substrate. Due to the coordinative properties of the macrocyclic substrate, we were able to detect and characterize the just-formed C(sp2)−OCH2CF3−Ni(II) species by mass-spectrometry and in-situ infrared spectroscopy of the mass-selected ion in the gas-phase.
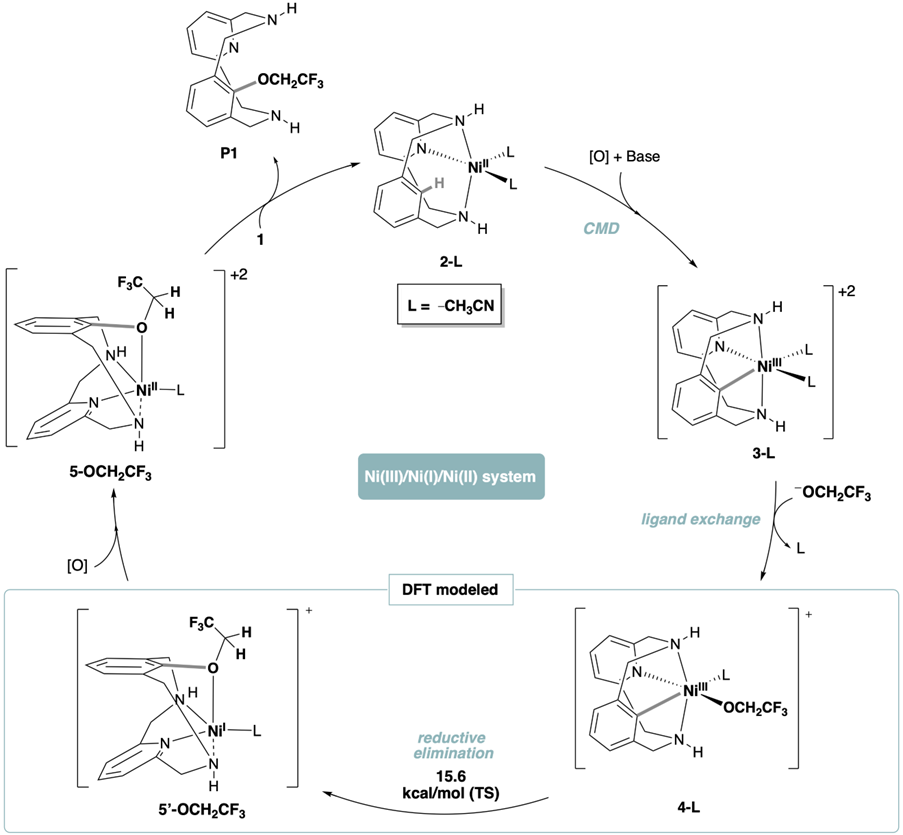
With this solid piece of mechanistic information in hand, DFT studies on the C(sp2)−OCH2CF3 bond formation mechanism indicate that the process involves a Ni(III)/Ni(I) reductive elimination followed by oxidation to Ni(II), rather than the energetically more demanding Ni(IV)/Ni(II) reductive elimination. This mechanistic investigation highlights the versatile redox abilities of Ni compounds and might help in designing new catalysts for the trifluoro- and 2,2-difluoro-ethoxylation of arene C−H bonds to synthesize bioactive fluorinated aryl-alkyl ethers.
Why are you doing this?
The limitation of traditional ether synthesis methods lies primarily in the need for pre-functionalized alkyl or aryl halide starting materials in methods like Williamson ether synthesis, Buchwald-Hartwig couplings, or Ullmann reactions. Therefore, finding a convenient strategy for the direct ethoxylation of C−H bonds is highly desired.
Indeed, in the context of drug discovery, the introduction of F atoms is key to boosting the stability and, thus, the efficiency of new drugs by preventing their fast decomposition in all stages of human metabolism, especially in the liver. Currently, 20% of all commercialized drugs contain fluorine. In addition, fluorination with F− anions or F+ oxidants usually needs delicate care, so the use of readily available solvents such as TFE and DFE to directly introduce the fluorinated motives is highly desirable.
Moreover, cheap Ni catalysts are largely unexplored in this transformation, so gaining mechanistic insights into the trifluoro- and difluoroethoxylation of C(sp2)−H bonds using nickel(II) was our driving force to undertake this study.
What is new and cool about your study?
This study reveals important mechanistic insights into the trifluoro- and difluoroethoxylation of C(sp2)−H bonds using nickel(II), and underscores the relevance of the redox promiscuity of Ni species. These balance from Ni(II) to Ni(III) and Ni(I), and combine 1-electron and 2-electron steps within the same catalytic cycle.
What is the main significance of your results?
The most important points are the description of the trifluoro- and difluoroethoxylation of C(sp2)−H bonds using nickel(II) complexes with a model macrocyclic arene substrate and common solvents such as TFE and DFE. The process itself may have limited practical use, but it is of relevance to unravel the precise mechanistic steps involved in the C−H ethoxylation and Ni-redox manifold in play.
The detection and characterization of the just-formed C(sp2)−OCH2CF3−Ni(II) species by mass spectrometry (MS) and in-situ IR spectroscopy are key to solving the puzzle.
Clarifying a precise mechanism with DFT calculations (see figure below), which involves the oxidation of Ni(II) to Ni(III), C−H activation bthrough a Concerted Metallation Deprotonation (CMD) reaction, reductive elimination, and re-oxidation to Ni(II), is valuable for the future development of Ni(I)/Ni(III) catalytic processes. This is particularly relevant for the 2,2,2-trifluoroethoxylation and 2,2-difluoroethoxylation of arene C−H bonds in the context of discovering improved methodologies to synthesize new F-containing bioactive compounds.
What is the benefit for using this finding in pharmaceutical research?
In the context of pharmaceutical research, Ni-catalyzed direct C−H functionalization to create ready-to-use F-containing drugs based on trifluoro- and difluoroethoxylation using common trifluoroethanol (TFE) and difluoroethanol (DFE) will be a highly desirable process. This process might be applied in the straightforward synthesis of new drugs and uses a highly available and reactive metal.
What part of your work was the most challenging?
One of the most challenging parts of this work was finding the system where some kind of intermediate species can be trapped, to have a solid experimental hint of the mechanism of the reactions. Once this was found, in this case by trapping an in-situ formed Ni(II)(Aryl−OCH2CF3) by ESI-MS, then the next challenge was to identify this species spectroscopically. This was done by performing in-situ gas-phase Infrared spectroscopy of the mass-selected and isolated ion with a technique developed by Professor Roithová, known as He-tagging IRPD.
Then, with this piece of solid mechanistic information, we worked out a DFT-verified catalytic cycle for the whole process led by Dr. Josep M. Luis, exploring the different options and selecting the most energetically favorable one.
The whole study was a puzzle that cannot be reliably sorted out without the original identification of the trapped intermediate species, which was possible thanks to the congested coordinative properties of the model macrocyclic ligand.
The use of macrocyclic model arene-containing ligand platforms has been key to trapping an actual intermediate of the reaction and thus to building all the mechanistic understanding of the process upon it. The macrocyclic ligand strategy has been very successful in the past in our group for unraveling proof-of-concept mechanistic details in C−H functionalizations or C−X cross-couplings using Ni and also other metals such as Co, Cu, Ag, and Au.
Thank you very much for these insights.
The article they talked about
- Sirect 2,2,2-Trifluoro and 2,2-Difluoroethoxylation of a Model Macrocyclic Ar−H Substrate via Ni-Catalysis,
Lorena Capdevila, Max T. G. M. Derks, Marc Montilla, Josep M. Luis, Jana Roithová, Xavi Ribas,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400023
Xavi Ribas is a Full Professor of Chemistry at the Institute of Computational Chemistry and Catalysis (IQCC), University of Girona, Spain.