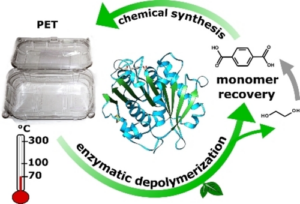
Polyethylene terephthalate, PET, is the most widely used plastic worldwide. Enzymatic recycling of PET components offers an economically and ecologically appealing alternative to incineration, landfilling, or purely chemical recycling.
Wolfgang Zimmermann, Norbert Sträter, and Christian Sonnendecker, Leipzig University, Germany, and colleagues have investigated the structure and functionality of the metagenomic-derived polyester hydrolase PHL7 and increased its efficiency. PHL7 is a thermophilic polyester hydrolase that, along with six homologs (PHL1-6), was isolated from a plant compost metagenome. It can quickly hydrolyze amorphous PET found in post-consumer plastic waste at 70 °C, producing terephthalic acid (TPA) and ethylene glycol (EG) [1]. The process initially generates mono-(2-hydroxyethyl) terephthalic acid (MHET), which is then further hydrolyzed to TPA and EG. PHL7 is considered a promising candidate for developing biocatalytic recycling processes for PET waste.
The researchers studied the binding mode of TPA to PHL7 and found that PHL7 interacts with the TPA moiety of its substrate in a lock-and-key mechanism rather than an induced fit. The main binding contributor is likely subsite I, which interacts with one PET moiety to achieve a productive conformation at the catalytic serine. Subsite II predominantly contributes to the initial substrate binding and guidance of the PET chain towards the active site. This model explains why MHET is formed as the main product, while release of TPA may predominantly occur as a subsequent reaction.
The researchers mutated amino acids of the active site and received PHL7 variants with changed PET-hydrolytic activity and thermal stability. The substrate-binding mode of TPA is similar to that of the thermophilic polyester hydrolase LCC and deviates from the mesophilic IsPETase. The subsite I modifications L93F and Q95Y, derived from LCC, increased the thermal stability, while exchange of H185S, derived from IsPETase, reduced the stability of PHL7. The subsite II residue H130 is suggested to represent an adaptation for high thermal stability, whereas L210 emerged as the main contributor to the observed high PET-hydrolytic activity. Variant L210T showed significantly higher activity, achieving a degradation rate of 20 µm h−1 with amorphous PET films.
According to the researchers, this study provides a structural basis for further systematic enhancement of the catalytic activity of polyester hydrolases.
- Structure and function of the metagenomic plastic-degrading polyester hydrolase PHL7 bound to its product,
P. Konstantin Richter, Paula Blázquez-Sánchez, Ziyue Zhao, Felipe Engelberger, Christian Wiebeler, Georg Künze, Ronny Frank, Dana Krinke, Emanuele Frezzotti, Yuliia Lihanova, Patricia Falkenstein, Jörg Matysik, Wolfgang Zimmermann, Norbert Sträter, Christian Sonnendecker,
Nature Communications 2023, 14, 1905
https://doi.org/10.1038/s41467-023-37415-x
[1] Christian Sonnendecker, Juliane Oeser, P. Konstantin Richter, Patrick Hille, Ziyue Zhao, Cornelius Fischer, Holger Lippold, Paula Blázquez-Sánchez, Felipe Engelberger, César A. Ramírez-Sarmiento, Thorsten Oeser, Yuliia Lihanova, Ronny Frank, Heinz-Georg Jahnke, Susan Billig, Bernd Abel, Norbert Sträter, Jörg Matysik, Wolfgang Zimmermann, Carbon Footprint Recycling of Post-Consumer PET Plastic with a Metagenomic Polyester Hydrolase, ChemSusChem 2021. https://doi.org/10.1002/cssc.202101062