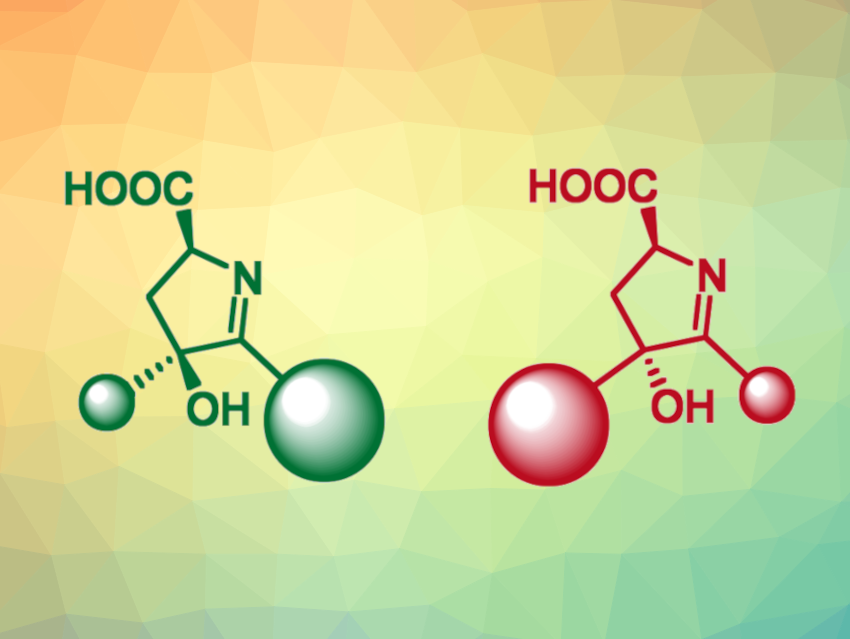
Aldolases are C−C bond-forming enzymes that can be useful in asymmetric organic synthesis. The preparation of enantiomerically pure products can be particularly important in pharmaceutical chemistry and agrochemistry. Protein engineering can be used to tune the enantioselectivity of target enzymatic reactions in biocatalytic processes.
Rui Zhang, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Xiang Sheng, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, University of the Chinese Academy of Sciences, Beijing, National Center of Technology Innovation for Synthetic Biology, Tianjin, China, Cangsong Liao, Shanghai Institute of Materia Medica and University of the Chinese Academy of Sciences, and colleagues have semirationally engineered a decarboxylative aldolase, obtaining an enzyme mutant that can be used for the divergent synthesis of cyclic imino acids (general product structures pictured) in high yields and selectivities.
The team used the enzyme UstD, which usually catalyzes decarboxylative aldol reactions between L-aspartate and different electrophiles, and aimed to modify the active site of UstD to tune the stereoselectivity and regioselectivity, providing access to cyclic imino acid scaffolds. The researchers used a focused rational iterative site-specific mutagenesis (FRISM) strategy to optimize the enzyme. They created a small library of mutants, targeting three key residues in the active site.
Using this approach, the team ultimately obtained an engineered enzyme that showed excellent selectivity and a broad substrate scope for reactions of L-aspartate with vicinal diones or keto esters. They obtained 30 different cyclic imino acids using the engineered variant, with complementary regioselectivity and stereoselectivity to the wild-type UstD.
- Semirational Protein Engineering of a Decarboxylative Aldolase for Regiodivergent and Stereodivergent Synthesis of Cyclic Imino Acids,
Yuqiu Lan, Chenghua Zhang, Chunping Tang, Yang Ye, Rui Zhang, Xiang Sheng, Cangsong Liao,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202500080