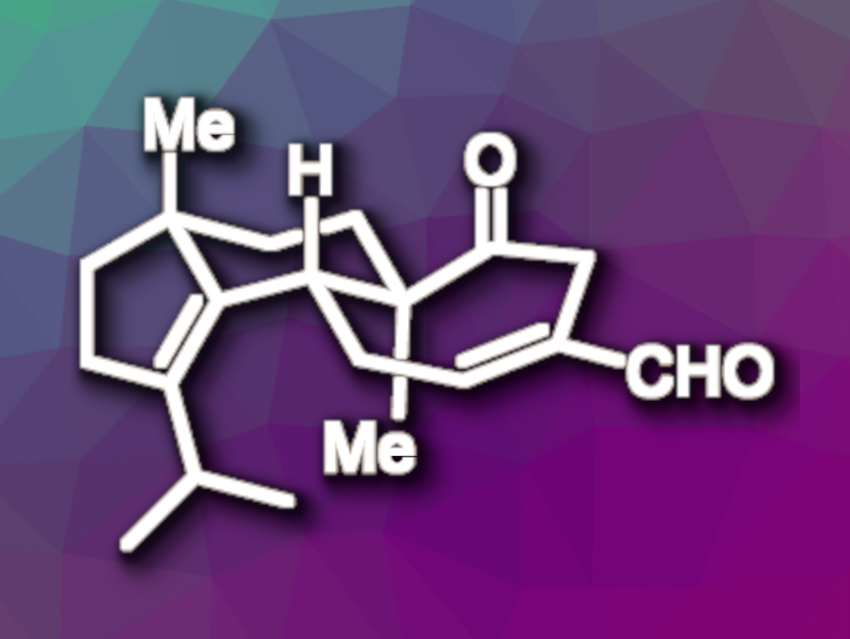
Cyathin B2 (pictured) is a natural product and an example of a cyathane diterpene. This type of molecule features a 5-6-7 tricyclic structure, and several of the compounds have interesting biological activities, such as antimicrobial, cytotoxic, or anti-inflammatory properties.
Yun Li, Lanzhou University, China. and colleagues have developed a method for the asymmetric total synthesis of (−)-cyathin B2 that is concise and could also be useful to provide strategies for the construction of other cyathane diterpenes and related compounds. The team used a platinum-catalyzed asymmetric diboration/desymmetric double-allylboration cascade to prepare the key intermediate, a fused 5-6 bicyclic system in an enantioselective manner. To achieve this, they reacted a prochiral cyclopentanedione derivative with a diene in the presence of a platinum catalyst with a bulky, chiral phosphine ligand and bis(pinacolato)diboron.
The researchers were able to scale up this reaction to the 30 g scale. The resulting bicyclic intermediate was functionalized via a conjugate addition using isopropylmagnesium and underwent several oxidation and reduction steps. Finally,another alkene side chain was introduced, and the seven-membered ring was closed using ring-closing metathesis.
Overall, the team synthesized (−)-cyathin B2 in 14 steps starting from commercially available materials. The cascade reaction used to prepare the fused 5-6 bicyclic system can also be used for a range of other substrates.
- Enantioselective Total Synthesis of (−)-Cyathin B2: A Desymmetric Double-Allylboration Approach,
Jianping Wang, Jiacheng Yin, Hayatullah Imtiaz, Hongyu Wang, Yun Li,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c08042