Chiral cyclobutanes are found, e.g., in natural products and bioactive compounds. The regio- and enantioselective synthesis of cyclobutane derivatives can be challenging. One approach to this is the visible-light-induced asymmetric [2 + 2] cycloaddition of alkenes. However, this type of reaction can require directing groups, and thus, multiple steps. New directing-group-free enantioselective [2 + 2] photocycloaddition reactions that give chiral cyclobutanes would be useful.
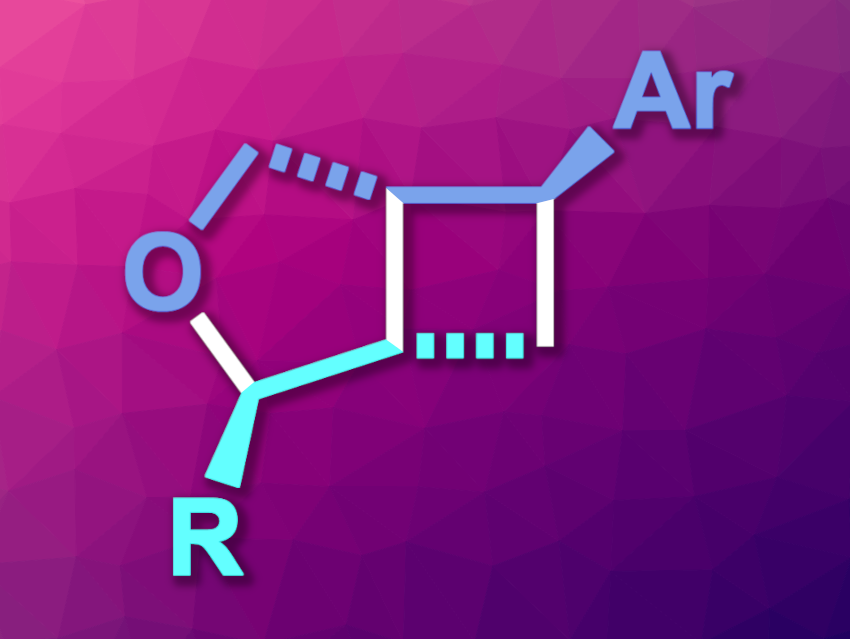
Shu-Li You, Shanghai Institute of Organic Chemistry, University of the Chinese Academy of Sciences, and colleagues have developed a method for the synthesis of enantioenriched cyclobutane derivatives (oxa-[3,2,0]-bicyclic heptanes, general structure pictured) that proceeds in a cascade fashion. The approach is based on an Ir-catalyzed asymmetric allylic etherification, followed by a visible-light-induced [2 + 2] cycloaddition.
The team reacted a range of cinnamyl alcohols with different allyl acetates, using [Ir(cod)Cl]2 (cod = 1,5-cyclooctadiene) together with a chiral phosphoramidite-based ligand as the catalyst for the asymmetric allylic etherification step, 3,5-Cl2C6H3CO2H as an acid additive, Ir(dFppy)3 (dFppy = 3,5-difluoro-2-(2-pyridinyl)phenyl) as a photosensitizer, and toluene as the solvent. The reactions were performed at room temperature under blue LED light.
Under these conditions, a wide range of oxa-[3,2,0]-bicyclic heptanes were obtained in moderate to high yields with good diastereoselectivities and excellent enantioselectivities. The team also performed a gram-scale reaction and obtained the desired product in a yield of 77 %. The method has a broad substrate scope, uses alkene substrates without directing groups, and is operationally simple due to the simultaneous addition of all starting materials and catalysts, without a need for the isolation of the ether intermediates.
- Enantioselective Synthesis of Cyclobutane Derivatives via Cascade Asymmetric Allylic Etherification/[2 + 2] Photocycloaddition,
Pusu Yang, Rui-Xiang Wang, Xu-Lun Huang, Yuan-Zheng Cheng, Shu-Li You,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c08792



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)