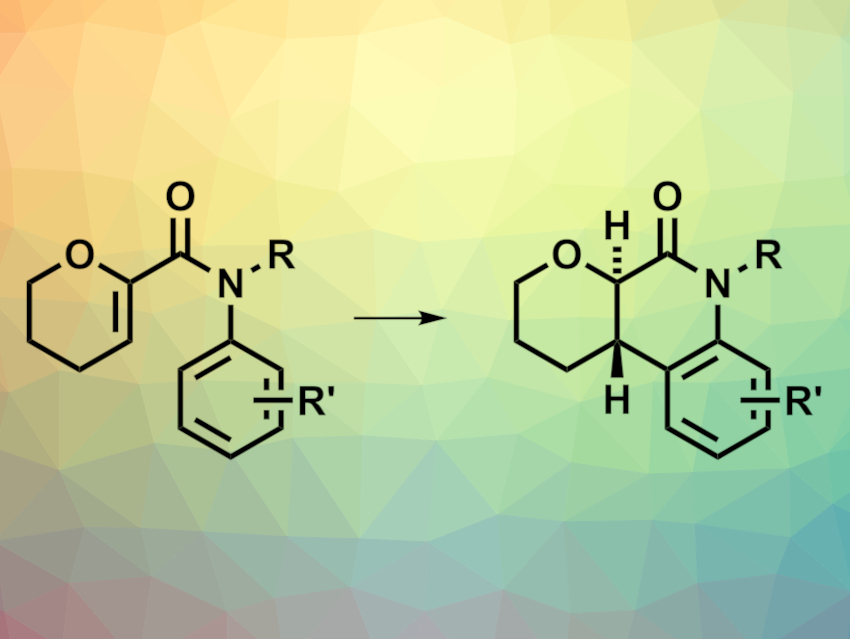
Photocyclization reactions are useful tools in organic synthesis. 6π Photocyclizations, for example, can be used for the synthesis of aliphatic heterocycles. However, these reactions tend to require UV light, which limits their scope. Catalytic photosensitizers can be used to enable transformations under visible light. Enantioselective variants, however, can still be challenging to achieve.
Robert Paton, Colorado State University, Ft. Collins, USA, Martin D. Smith, University of Oxford, UK, and colleagues have developed a catalytic enantioselective 6π photocyclization of acrylanilides to give substituted dihydroquinolones (pictured). The reactions use chiral Lewis acid complexes, which allow for enantioselective triplet-state reactions. The team used a 440 nm LED as the light source, Ir((5-CF3)(4′-t-Bu)ppy)3 as the photocatalyst (ppy = 2-phenylpyridine), scandium(III) triflate as the Lewis acid together with a chiral Feng ligand, and CH2Cl2 as the solvent. The reactions were performed at 10–15 °C.
The desired dihydroquinolone derivatives were obtained in moderate to high yields and with mostly high stereoselectivities. According to the team, the yield and enantioselectivity of the reaction depend heavily on the photocatalyst, and the work could be useful for the development of other Lewis acid-mediated enantioselective triplet-sensitized reactions.
- Catalytic Enantioselective 6π Photocyclization of Acrylanilides,
Benjamin A. Jones, Pearse Solon, Mihai V. Popescu, Ji-Yuan Du, Robert Paton, Martin D. Smith,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c09267