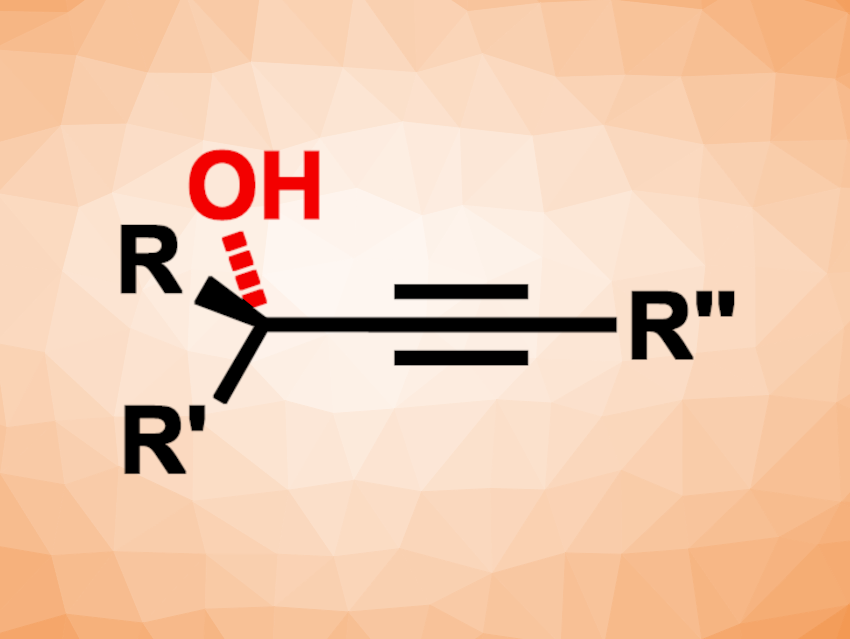
The functionalization of C–H bonds with oxygen-containing groups is an important type of transformation in organic synthesis. Enantioselective hydroxylations that give chiral alcohols, for example, are useful. They can be catalyzed by enzymes, and there are also examples of small molecules that serve as chiral catalysts. However, these synthetic catalysts for the asymmetric hydroxylation of C–H bonds can have limited substrate scopes, e.g., restricted to cyclic systems. In addition, the preparation of enantioenriched tertiary alcohols via such a pathway can be particularly challenging due to, e.g., a tendency for racemization.
Lei Liu, Shandong University, Jinan, China, and Shenzhen Research Institute of Shandong University, Shenzhen, China, and colleagues have developed a method for the enantioselective hydroxylation of tertiary propargylic C–H bonds in acyclic molecules (general product structure pictured). The team used a manganese-based catalyst with a bulky, chiral salen-type ligand that combines chiral carbon centers with the axial chirality of biaryl group substituents. Iodosylbenzene (PhIO) was used as an oxidant and CH3NO2 as the solvent. The reactions were performed at –20 °C.
Under these conditions, one enantiomer of a wide range of racemic alkyne substrates was hydroxylated, giving the desired hydroxylation products in yields of up to 50 % from the racemate with high enantioselectivity, as well as the remaining enantiopure alkyne. The remaining alkyne can be recovered and oxidized to the corresponding tertiary alcohol. Thus, the work provides access to both enantiomers of the alcohols.
- Catalytic Enantioselective Hydroxylation of Tertiary Propargylic C(sp3)–H Bonds in Acyclic Systems: a Kinetic Resolution Study,
Min Cao, Hongliang Wang, Fangao Hou, Yuhang Zhu, Qianqian Liu, Chen-Ho Tung, Lei Liu,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c03610