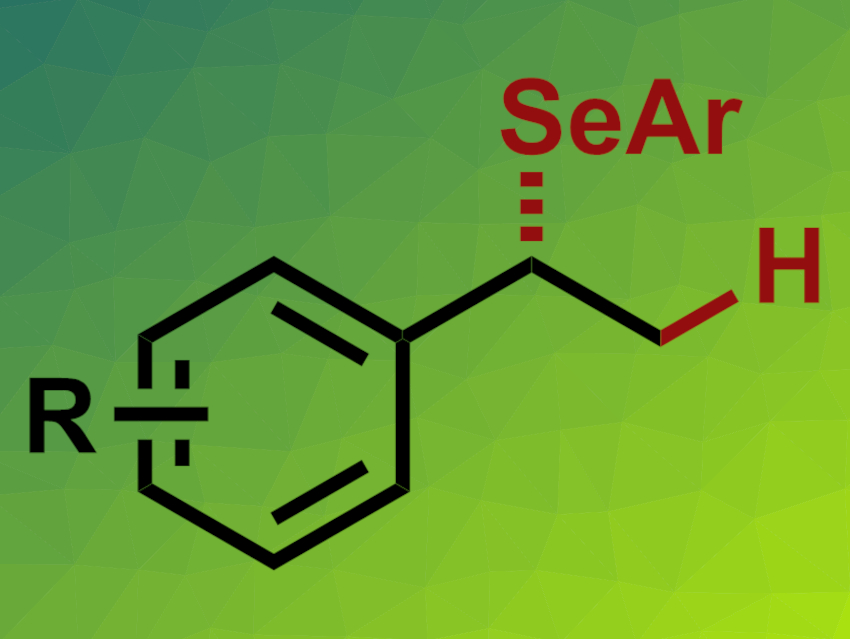
Organoselenium compounds are generally less well-studied than their sulfur or oxygen counterparts. Hydroselenation reactions could be used for the synthesis of such organoselenium compounds in an atom-economical manner. Enantioselective variants of this type of reaction would be particularly interesting.
Vy M. Dong, University of California, Irvine, USA, Xiao-Hui Yang, Beijing Institute of Technology, China, and colleagues have achieved the first enantioselective hydroselenation of styrenes (general product structure pictured). The team used various alkenes and selenols as substrates, Rh(cod)2BF4 as a catalyst together with a commercially available, chiral, biphenyl-based diphosphine ligand, and 1,2-dicholorethane (DCE) as the solvent. The reactions were performed at 30 °C.
The desired hydroselenation products were obtained in moderate to high yields and with mostly good enantioselectivities. The reaction is selective for the Markovnikov product, i.e., branched isomers. The team proposes a mechanism that involves a Rh-hydride species, which coordinates the alkene. This is followed by a migratory insertion of the alkene into the Rh–H bond, reductive elimination, and dissociation of the product. According to the researchers, the results could be helpful for future work on the functionalization of alkenes using chalcogen nucleophiles.
- Enantioselective Selenol-ene Using Rh-Hydride Catalysis,
Hannah S. Slocumb, Shaozhen Nie, Vy M. Dong, Xiao-Hui Yang,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08475