Enantioselective multicomponent reactions can be used to create complex organic molecules in a single step. Achieving high selectivities in this type of transformation, however, may be challenging. Combining radical transformations with metal catalysis (“radical-polar crossover”) can be useful in this context, as it can utilize both the reactivity of radicals and the selectivity of metal-catalyzed reactions.
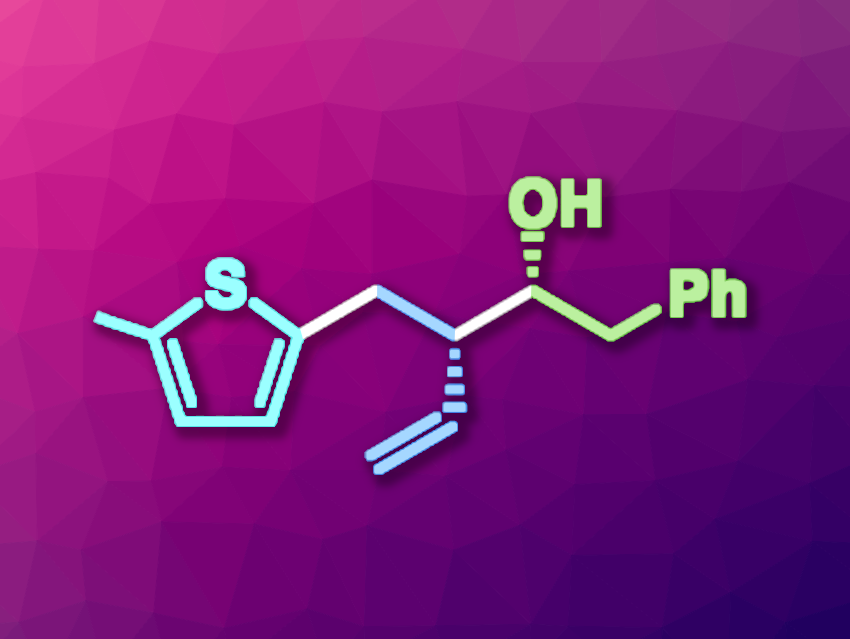
Huan-Ming Huang, ShanghaiTech University, Shanghai, China, and colleagues have developed an enantioselective, three-component radical-polar crossover transformation for the (hetero)arylalkylation of 1,3-dienes (example product pictured) that uses both photoredox and chromium catalysis. This reaction has a broad substrate scope, including drug-like building blocks, and generates two chiral centers in one step.
The team reacted different (hetero)arenes with a range of 2-substituted 1,3-dienes or 1,3-butadiene and a variety of aliphatic or aromatic aldehydes. They used an acridinium-based photoredox catalyst together with CrCl2 and a chiral bisoxazoline ligand. The reactions were performed under 450 nm LED light at room temperature. Under these conditions, the desired products were obtained in moderate to excellent yields with generally high enantioselectivities.
The researchers propose a mechanism that involves the photocatalytic generation of a radical cation from the (hetero)arene, which is trapped by the diene, followed by deprotonation. The resulting allylic radical intermediate then reacts with the aldehyde component under Cr catalysis. According to the team, the combination of chiral chromium catalysis with photoredox catalysis could lead to further developments in enantioselective radical-polar crossover chemistry.
- Photoredox/Cr-catalyzed enantioselective radical-polar crossover transformation via C–H functionalization,
Si-Yuan Tang, Zhan-Jie Wang, Yu Ao, Ning Wang, Huan-Ming Huang,
Nat. Commun. 2025.
https://doi.org/10.1038/s41467-025-56372-1