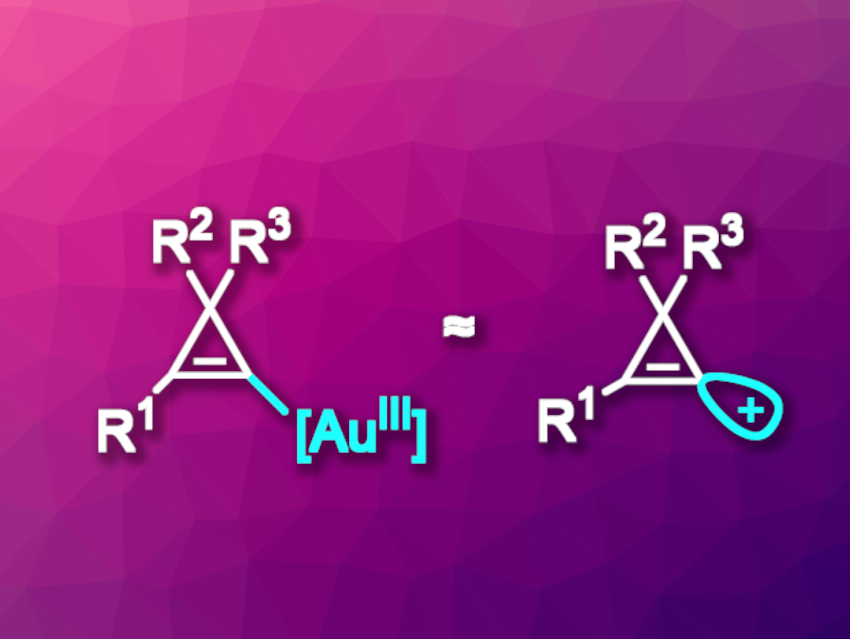
Cyclopropenes are the smallest cyclic alkenes. Their high reactivity is due to the substantial ring strain. Cyclopropenium cations generated by removing a substituent from the “saturated” corner of the ring (π-type cations) are aromatic, which makes the cyclopropenes easily ionizable. In contrast, formally removing a substituent from the carbon atoms involved in the double bond would give an unstable “σ-type” cation, in which the positive charge remains localized. Due to this instability, free σ-type cations generally cannot be used as precursors in organic synthesis, and synthetic equivalents of these elusive species can be useful.
Jérôme Waser, Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland, and colleagues have developed a method for the preparation of highly functionalized alkynyl- or alkenyl-cyclopropenes that uses electrophilic cyclopropenyl-gold(III) species as synthetic equivalents of σ-type cyclopropenium cations (general structures pictured). The electrophilic species are created by first converting the cyclopropene substrates to cyclopropenyl benziodoxoles, a hype of hypervalent species. This is followed by an oxidative addition to the gold catalyst. The team used (Me2S)AuCl as the catalyst together with 1,10-phenanthrolin-5,6,-dione as a ligand.
The cyclopropenyl-gold(III) species prepared in situ are then reacted with terminal alkynes or vinylboronic acids to give the desired functionalized cyclopropenes. Good to excellent yields were obtained for the alkynyl-functionalized products and moderate to high yields for the alkenyl-substituted ones. Due to the mild reactions conditions, various functional groups are tolerated, and the reaction can be used for late-stage functionalizations. The products can be further functionalized to give other cyclopropenes, cyclopropane derivatives, or conjugated enynes (via ring opening).
- Accessing elusive σ-type cyclopropenium cation equivalents through redox gold catalysis,
Xiangdong Li, Matthew D. Wodrich, Jérôme Waser,
Nat. Chem. 2024, 16, 901–912.
https://doi.org/10.1038/s41557-024-01535-8