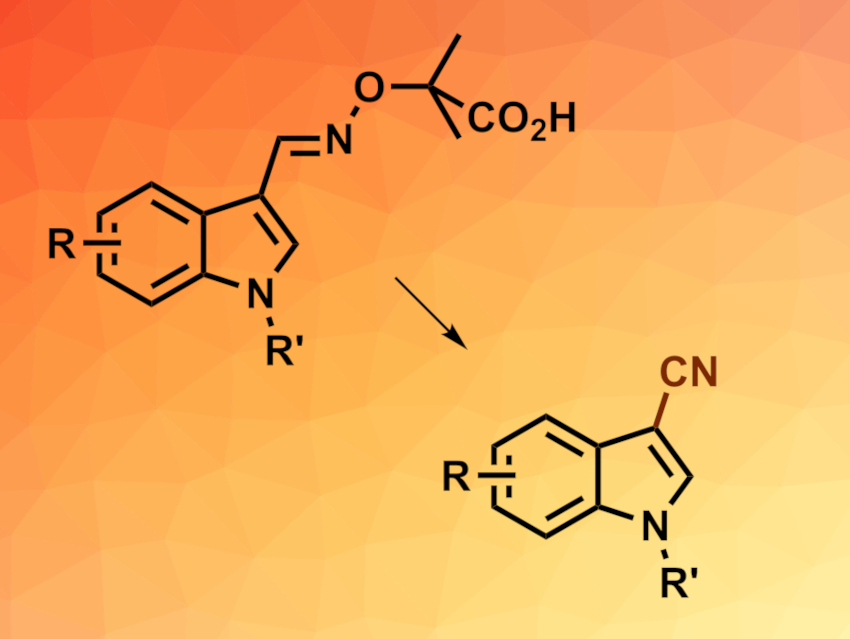
Aryl or heteroaryl nitriles can be useful intermediates in organic synthesis and are also found, e.g., in natural products or agrochemicals. Methods for their synthesis are, thus, interesting research targets. α-Imino-oxy acids (example pictured above on the left), a type of oxime ether, can undergo transformations into the corresponding iminyl radicals (R2C=N•). Such iminyl radicals can be useful for the synthesis of different nitrogen-containing compounds.
Jing-Mei Huang, South China University of Technology, Guangzhou, China, and colleagues have developed a method for the electrochemical oxidative decarboxylation of α-imino-oxy acids to give (hetero)aryl nitriles via iminyl radicals (overall reaction pictured). The team first prepared different α-imino-oxy acids from indoles via 1H-indole-3-carbaldehyde intermediates. These aldehydes were reacted with 2-(aminooxy)-2-methylpropanoic acid hydrochloride to give the desired substrates.
The resulting α-imino-oxy acids were then subjected to electrolysis in an undivided cell with a reticulated vitreous carbon (RVC) anode, a platinum cathode, tetrabutylammonium acetate (TBAOAc) as the electrolyte, and a mixture of methanol and acetonitrile as the solvent. The corresponding nitriles were obtained in moderate to excellent yields. Both electron-donating and electron-withdrawing substituents on the indole rings are tolerated. The team demonstrated the usefulness of the method with a one-pot, gram-scale conversion of 1-methyl-1H-indole-3-carbaldehyde to the corresponding nitrile.
- Electrosynthesis of (hetero)aryl nitriles from α-imino-oxy acids via oxidative decarboxylation/N–O cleavage,
Hui-Shan Lin, Shu-Jun Chen, Jing-Mei Huang,
Chem. Commun. 2022.
https://doi.org/10.1039/D2CC02986C