Gold nanoparticles (AuNPs) have unique physicochemical properties and surface functionalities. They are one of the most important nanomaterials with applications in optical sensing, chemical catalysis, and biomedicine. A challenge in the synthesis of nanoparticles is their tendency to aggregate, which can drastically change their physicochemical properties and interactions with other materials.
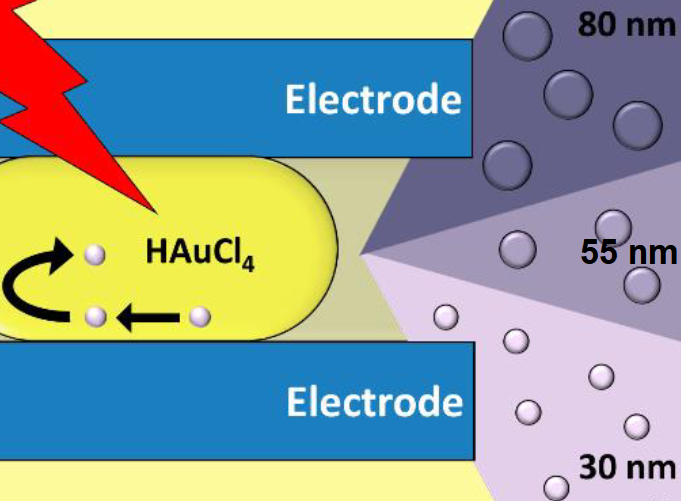
Mario Saucedo-Espinosa, Maximilian Breitfeld and Petra Dittrich, ETH Zurich, Basel, Switzerland have developed an approach to synthesize AuNPs. The researchers used a microfluidic device to create nanoliter droplets with an aqueous HAuCl4 solution, immersed in a hydrophobic phase. When the droplets passed through an electrical field between a pair of electrodes, Au(III) was reduced and AuNPs were formed.
Importantly, the solution inside the droplets was moved constantly, which prevents aggregation of the just formed AuNPs. The final size of the AuNPs was between 30 to 100 nm, as defined by the initial concentration of the metal precursor and droplet velocity.
The technique achieves precision synthesis of AuNPs without stabilizers and could be relevant for the synthesis of other metal nanoparticles, say the researchers.
- Continuous Electroformation of Gold Nanoparticles in Nanoliter Droplet Reactors,
M. A. Saucedo-Espinosa, Max Breitfeld, Petra S Dittrich,
Angewandte Chemie International Edition 2022.
https://doi.org/10.1002/anie.202212459