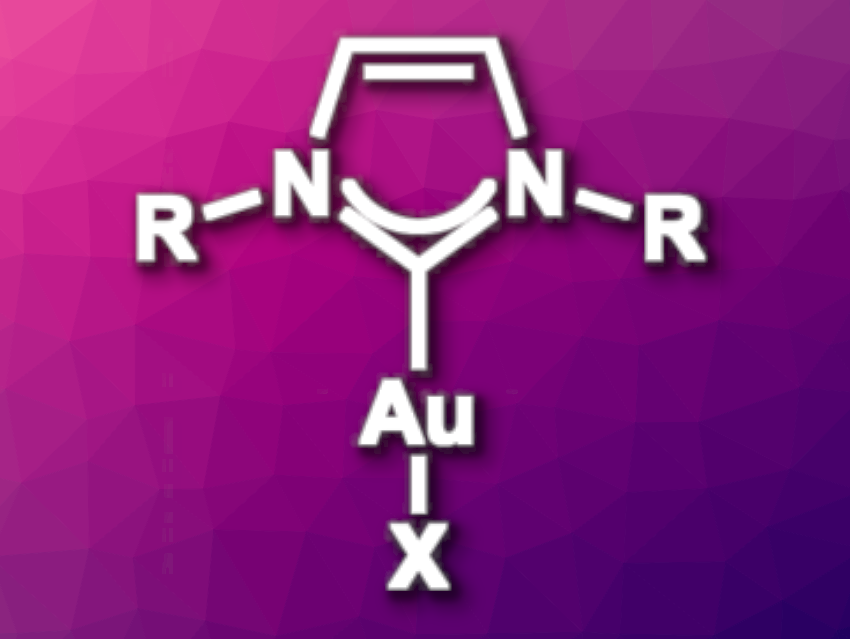
Gold N-heterocyclic carbene (Au-NHC) complexes are widely used in catalysis. They are generally synthesized either using a strong base to generate a free carbene or via a transmetalation route. However, these methods generate by-products that remain in the reaction mixture.
Thomas P. Nicholls, Justin M. Chalker, Flinders University, Australia, and colleagues have found that an electrochemical approach can be used to produce Au-NHC complexes without any by-products other than hydrogen gas. This allows the complexes to be used directly in catalytic reactions without any work-up or purification. A sacrificial gold anode releases Au(I) ions into solution and an imidazolium salt is reduced at the cathode to produce the NHC and hydrogen gas. The Au-NHC complex is the only species remaining in solution upon completion of the reaction.
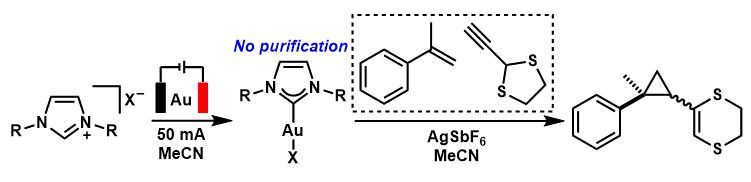
The generated Au-NHC complexes can then be used in catalysis. The team directly used the electrochemical reaction mixture in catalytic reactions and found that the Au-NHC catalysts performed similarly to the isolated catalysts. This method could be used to speed up reaction development by expediting catalyst screening, as there is no need for work-up or purification.
- Electrochemical synthesis of gold‐N‐heterocyclic carbene complexes,
Thomas Peter Nicholls, Zhongfan Jia, Justin M. Chalker,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202303161