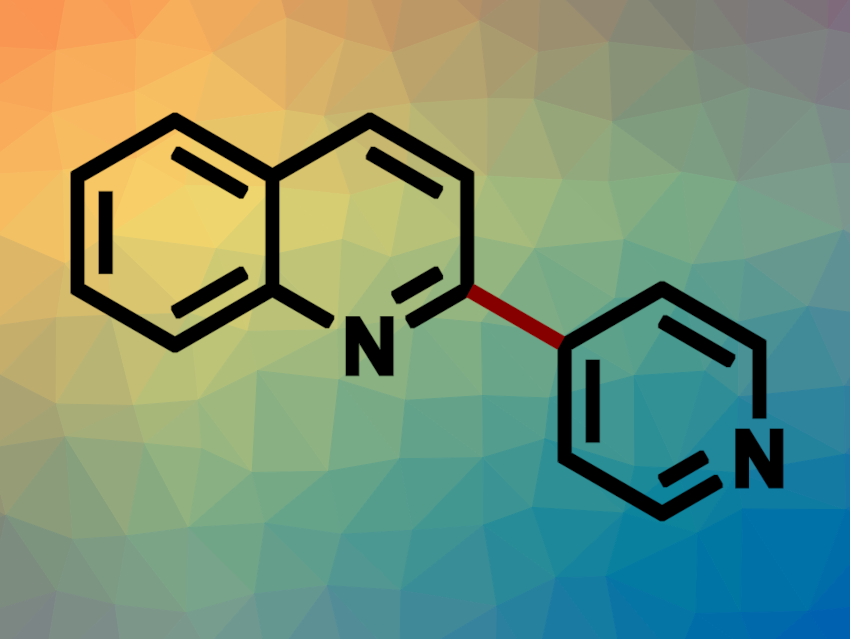
Biheteroaryls are structural motifs in organic compounds such as natural products, ligands, and pharmaceutically active compounds. They are often synthesized using transition-metal-catalyzed cross-coupling reactions. Alternative methods with wide substrate scopes that proceed under mild conditions and provide good regioselectivities are interesting research targets.
Shenlin Huang, Nanjing Forestry University, China, and colleagues have developed an electrochemical method for the synthesis of biheteroaryls (example pictured) via the coupling of N-heteroarenes with heteroaryl phosphonium salts. The team used a graphite anode, a zinc cathode, nBu4NBr as an electrolyte, and dimethyl sulfoxide (DMSO) as the solvent in an undivided electrochemical cell to react pyridylphosphonium triflates with N-heteroarenes in the presence of trifluoroacetic acid (TFA) under a constant current.
The desired biheteroaryl compounds were obtained in moderate to good yields. The team proposes a reaction mechanism that involves the protonation of the substrates by TFA, followed by cathodic reduction and a radical-radical coupling. The loss of PPh3 and a deprotonation step then give the desired product. According to the researchers, the adsorption of the radical intermediates to the zinc cathode might facilitate the radical-radical coupling.
- Cathodically Coupled Electrolysis to Access Biheteroaryls,
Tianyu He, Chaoqiang Liang, Haoyuan Cheng, Shuai Shi, Shenlin Huang,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.3c03859