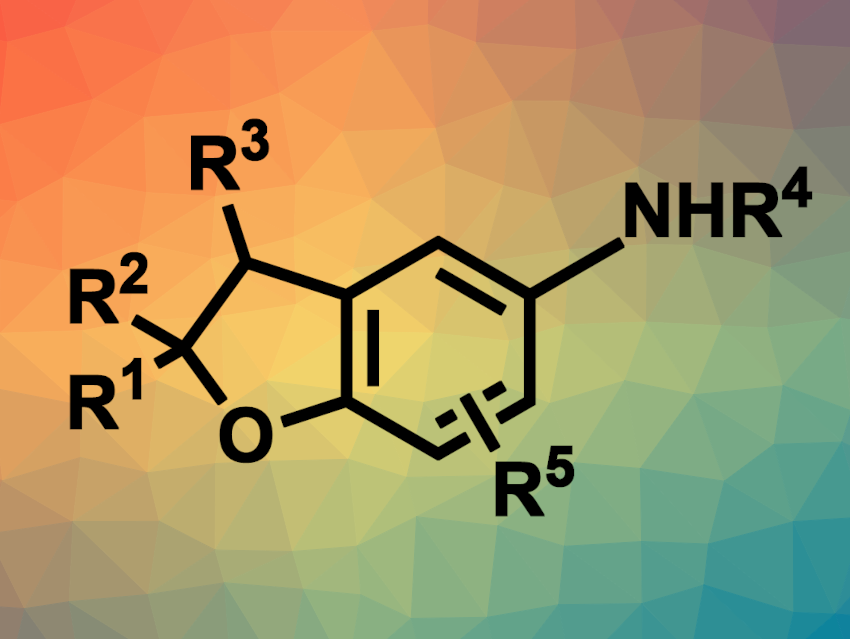
2,3-Dihydrobenzofuran, or coumaran, units are often found in natural products and pharmaceutically active compounds. 5-Aminocoumarans, in particular, have shown some interesting biological activities. Thus, the development of new methods for the synthesis of this type of compound is an interesting research target.
Yan-Hong He, Zhi Guan, Southwest University, Chongqing, China, and colleagues have developed a method for the electrochemical synthesis of 5-aminocoumaran derivatives from aminophenols and readily available alkenes. The team reacted a variety of styrene derivatives and other alkenes with a few different 4-aminophenol derivatives, using an undivided electrochemical cell with a carbon anode and a platinum cathode, acetonitrile as the solvent, and nBu4NBF4 as the electrolyte. The reactions were performed at room temperature under air with a constant current of 10 mA.
The desired 5-aminocoumarans were produced in moderate to excellent yields without a need for catalysts, additives, oxidants, or sacrificial reagents, and with hydrogen as the sole byproduct. The team proposes a mechanism that involves the anodic oxidation of the 4-aminophenol to form a radical intermediate. This intermediate undergoes addition to the alkene, followed by a second oxidation to give a carbocation and an intramolecular cyclization to give the desired product. At the cathode, protons are reduced to form H2.
- Electrosynthesis of 5-Aminocoumaran Derivatives from Olefins and Aminophenols,
Yu-Hao Yang, Yu-Fang Tan, Ya-Nan Zhao, Yan-Hong He, Zhi Guan,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03026


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
