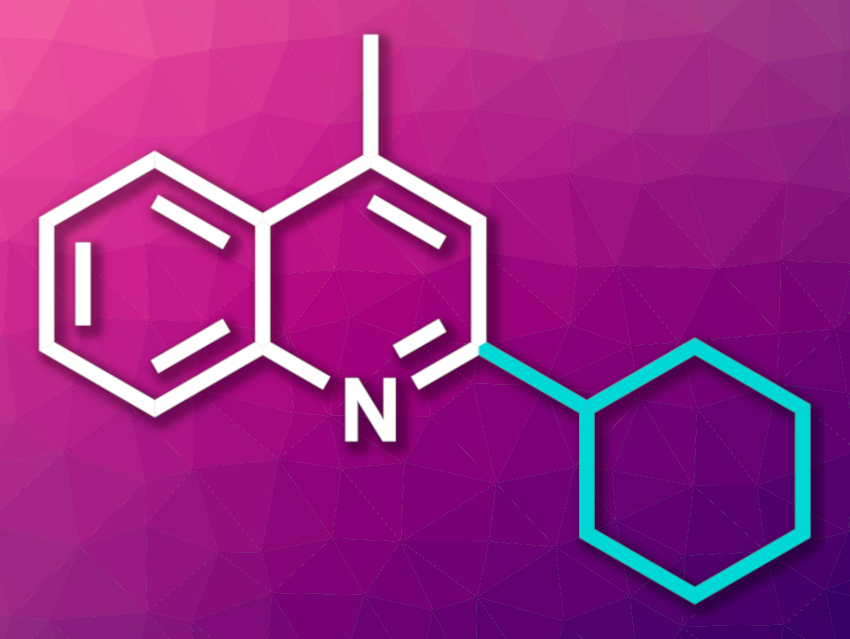
The Minisci reaction is a type of substitution at an electron-deficient aromatic compound, e.g., the introduction of an alkyl group to a nitrogen-containing heterocycle. New Minisci-type alkylation methods are interesting research targets. Many approaches require inert conditions, long reaction times, additives, or expensive photocatalysts. Electrochemistry could provide a simpler, more atom-economical path.
Jose A. Fernández-Salas, José Alemán, Universidad Autónoma de Madrid, Spain, and colleagues have developed a Minisci-type alkylation of N-heteroarenes (example product pictured) under electrochemical conditions using alkyl halides as reactants. The team reacted a range of heterocycles with primary, secondary, or tertiary alkyl iodides in the presence of diphenyl phosphate and NH4PF6, using a solvent system consisting of tetrahydrofuran (THF) and water in an undivided electrochemical cell with a carbon-based anode and a nickel-foam cathode.
The desired alkylated products were obtained in moderate to high yields. The reaction has a broad functional group tolerance, and the team used it in the formal syntheses of several bioactive compounds. The researchers propose a mechanism in which oxygen initiates the process, forming a superoxide anion. This anion is protonated to generate peroxy radical species, which could abstract a halogen atom from the alkyl halide and generate an alkyl radical. This radical can form the desired new carbon–carbon bond.
- General electrochemical Minisci alkylation of N-heteroarenes with alkyl halides,
Roberto del Río-Rodríguez, Lorena Fragoso-Jarillo, Alberto F. Garrido-Castro, M. Carmen Maestro, Jose A. Fernández-Salas, José Alemán,
Chem. Sci. 2022.
https://doi.org/10.1039/d2sc01799g