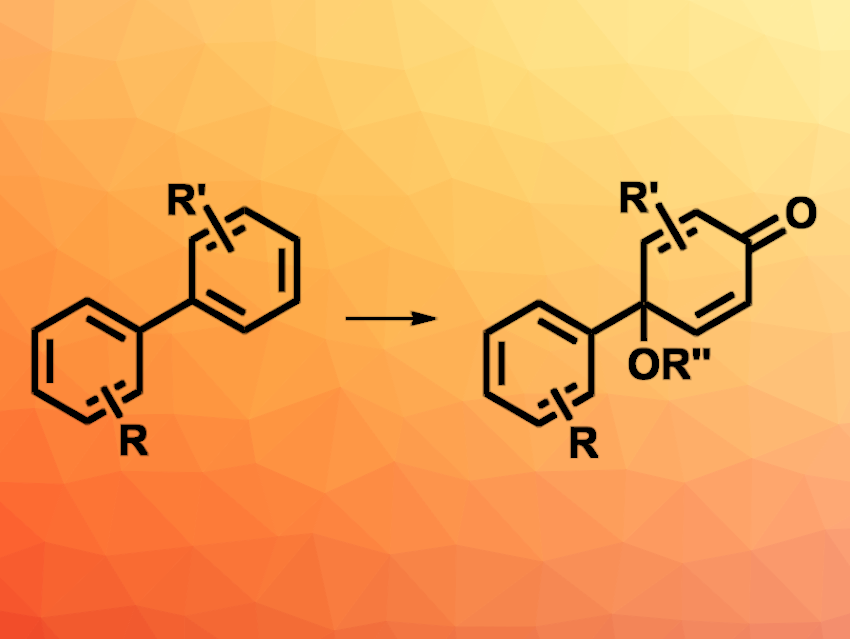
Cyclohexadienes are found, e.g., in some natural products and bioactive compounds. They are commonly synthesized via the oxidation of phenols. However, the synthesis of the phenol substrates usually requires several functionalization steps from arene feedstocks. Electrooxidative dearomatization reactions could provide a more direct alternative.
Jinbao Xiang, Jilin University, Changchun, China, and colleagues have developed an electrooxidative dearomatization of biphenyls for the synthesis of cyclohexadienones (pictured). The team transformed a variety of biphenyl derivatives into the corresponding cyclohexadienones using an undivided electrochemical cell with low-cost graphite and nickel foam electrodes, a mixture of water and methanol (or other alcohols) as the solvent, tetraethylammonium p-toluenesulfonate (Et4NOTs) as a supporting electrolyte, and AcOH as an acid. The reactions were performed at room temperature and with a current of 10 mA.
The desired cyclohexadienones were obtained in moderate to good yields and with high regioselectivities. The reaction tolerates a range of functional groups on the phenyl ring, including halogens, esters, carboxylic acids, as well as carbonyl, alkyl, or alkoxy groups. Some oxidation-sensitive functional groups were also tolerated, e.g., alcohols or alkenes. Due to the mild conditions, the reaction could also be useful for the late-stage modification of pharmaceutically active compounds. According to the researchers, the approach offers an attractive alternative to conventional methods for the synthesis of cyclohexadienone derivatives.
- Electrooxidative Dearomatization of Inactive Biphenyls to Cyclohexadienones,
Lingling Shi, Lianyou Zheng, Shulin Ning, Qiansong Gao, Chengcheng Sun, Zhuoqi Zhang, Jinbao Xiang,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02278