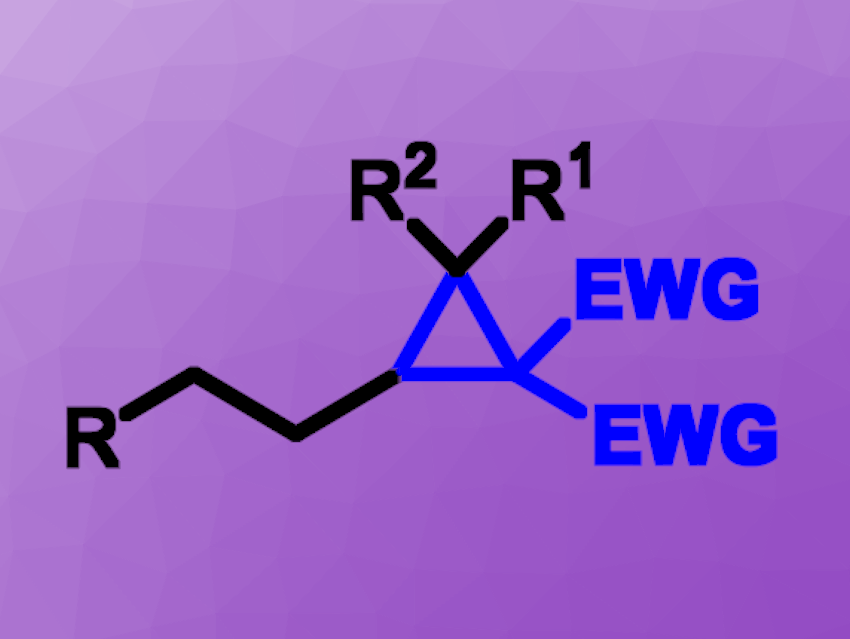
Cyclopropanes are common structural motifs in natural products, pharmaceuticals, and bioactive compounds. Efficient strategies for the synthesis of these three-membered rings from bench-stable starting materials with broad substrate scopes and good functional group tolerances are interesting research targets.
Youai Qiu, Nankai University, Tianjin, China, and colleagues have developed an electrochemical method for the direct cyclopropanation of unactivated alkenes using active methylene compounds. The team used an undivided cell with a graphite felt anode and a platinum plate cathode. The reactions were performed using catalytic amounts of ferrocene to promote the desired cyclopropanation, Et4NI as the electrolyte, and a mixture of dimethylformamide (DMF) and water as the solvent.
Under these conditions, the researchers reacted a variety of unactivated alkenes with active methylene compounds such as β-ketoesters and β-phosphonate esters. The desired products were obtained in moderate to high yields. The method was also employed for the late-stage functionalization of natural products. The team proposes a mechanism that involves the ferrocene-mediated generation of carbon-centered radicals from the methylene compounds.
- Electrochemical Cyclopropanation of Unactivated Alkenes with Methylene Compounds,
Min Liu, Yanwei Wang, Chao Gao, Jingpei Jia, Zile Zhu, Youai Qiu,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202425634