Quinolines are heteroaromatic compounds that feature fused benzene and pyridine rings. Quinoline derivatives are used, e.g., as pharmaceutically active compounds. Methods for the functionalization of quinolines are, thus, useful in medicinal chemistry, for example.
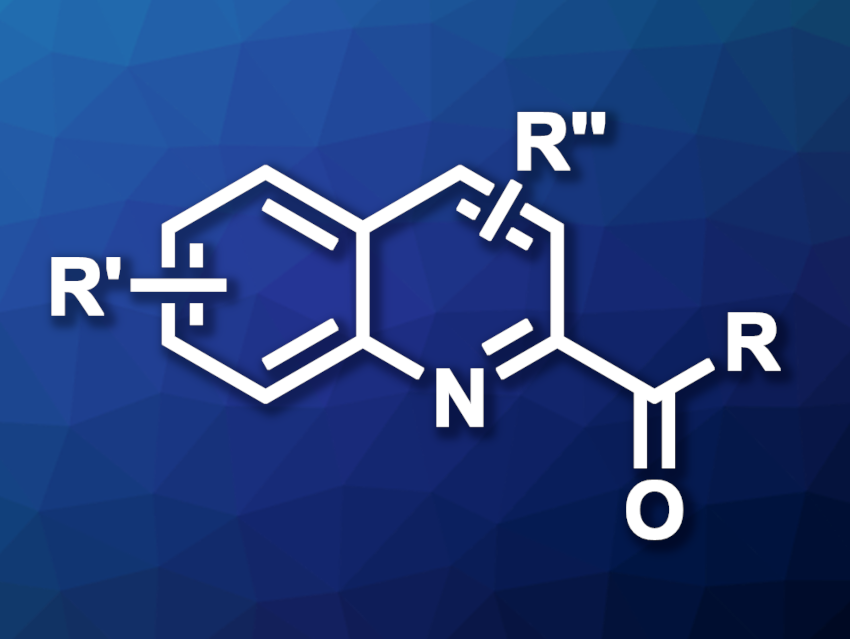
Ping Liu, Peipei Sun, Nanjing Normal University, China, and colleagues have developed an electrochemical hydrogen atom transfer (HAT) strategy for the C(sp2)–H acylation of quinolines and isoquinolines (example product structure pictured). The team used a series of quinolines or isoquinolines as substrates, alcohols as acyl sources (e.g., methanol for formylations), nBu4NBF4 as the electrolyte, p-toluenesulfonic acid (p-TsOH) as an acid additive, and (N-hydroxyphthalimide) (NHPI) as a HAT catalyst. The reactions were performed using constant current electrolysis in an undivided cell in MeCN at 60 °C, with a graphite felt (GF) anode and a platinum plate cathode under an air atmosphere.
The team obtained the desired acylated quinolines and isoquinolines in mostly moderate to high yields. Ethanol and 1-propanol as alcohol reagents were suitable for acetylation and propionylation reactions, respectively. The researchers also used the approach in a gram-scale experiment and obtained the desired product in a yield of 68 %. According to the team, the method could have applications in pharmaceutical chemistry.
- HAT-Mediated Electrochemical C(sp2)–H Acylation of Quinolines with Alcohols,
Yujie Liao, Cong Jiang, Congcong Qiang, Ping Liu, Peipei Sun,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02668



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)